在慢性应激模型中,抑制 MiR-186-5p 可恢复突触传递和神经元网络活动。
IF 9.6
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
慢性压力会对认知和情绪行为产生深远的负面影响,是导致神经精神疾病的一个主要风险因素。然而,慢性应激及其对神经元和突触功能的有害影响之间的分子联系仍然难以捉摸。在这里,我们采用体外和体内相结合的方法证明,慢性应激引发的 miR-186-5p 上调可能是这种变化的关键介质,从而导致突触功能障碍。我们的研究结果表明,miR-186-5p 的表达水平在长期暴露于应激的小鼠前额叶皮层(PFC)和长期暴露于地塞米松的皮层神经元中都有所增加。此外,在天真小鼠的前额叶皮层中病毒过表达 miR-186-5p 会诱发焦虑和抑郁样行为。在体外或小鼠慢性应激模型中,miR-186-5p 通过长时间的糖皮质激素受体激活而上调,会对谷氨酸能和 GABA 能突触传递产生不同影响,导致兴奋/抑制失衡,从而改变神经元网络活动。在谷氨酸能突触处,我们观察到突触 AMPARs 和突触传递均有所减少,而 GABA 能突触传递则有所加强。这些变化在体外可被 miR-186-5p 抑制剂所挽救。总之,我们的研究结果在慢性糖皮质激素受体激活、miR-186-5p 上调和慢性应激诱导的突触变化之间建立了一种新的分子联系,这种联系可能适合治疗干预。本文章由计算机程序翻译,如有差异,请以英文原文为准。

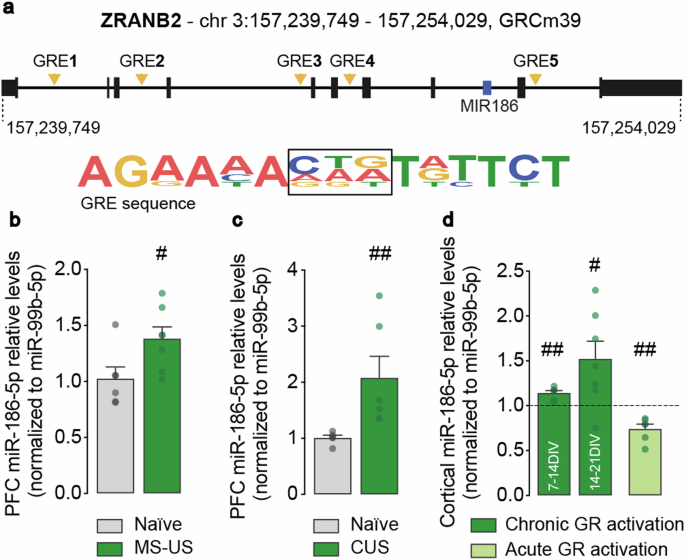
MiR-186-5p inhibition restores synaptic transmission and neuronal network activity in a model of chronic stress
Chronic stress exerts profound negative effects on cognitive and emotional behaviours and is a major risk factor for the development of neuropsychiatric disorders. However, the molecular links between chronic stress and its deleterious effects on neuronal and synaptic function remain elusive. Here, using a combination of in vitro and in vivo approaches, we demonstrate that the upregulation of miR-186-5p triggered by chronic stress may be a key mediator of such changes, leading to synaptic dysfunction. Our results show that the expression levels of miR-186-5p are increased both in the prefrontal cortex (PFC) of mice exposed to chronic stress and in cortical neurons chronically exposed to dexamethasone. Additionally, viral overexpression of miR-186-5p in the PFC of naïve mice induces anxiety- and depressive-like behaviours. The upregulation of miR-186-5p through prolonged glucocorticoid receptor activation in vitro, or in a mouse model of chronic stress, differentially affects glutamatergic and GABAergic synaptic transmission, causing an imbalance in excitation/inhibition that leads to altered neuronal network activity. At glutamatergic synapses, we observed both a reduction in synaptic AMPARs and synaptic transmission, whereas GABAergic synaptic transmission was strengthened. These changes could be rescued in vitro by a miR-186-5p inhibitor. Overall, our results establish a novel molecular link between chronic glucocorticoid receptor activation, the upregulation of miR-186-5p and the synaptic changes induced by chronic stress, that may be amenable to therapeutic intervention.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Psychiatry
医学-精神病学
CiteScore
20.50
自引率
4.50%
发文量
459
审稿时长
4-8 weeks
期刊介绍:
Molecular Psychiatry focuses on publishing research that aims to uncover the biological mechanisms behind psychiatric disorders and their treatment. The journal emphasizes studies that bridge pre-clinical and clinical research, covering cellular, molecular, integrative, clinical, imaging, and psychopharmacology levels.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


