嗅球中的饥饿信号为探索、寻找食物和外周新陈代谢提供了动力。
IF 7
2区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
摘要
研究目的虽然生物体的新陈代谢状态会影响嗅觉功能,但其确切机制及其对行为和新陈代谢的影响仍不清楚。在此,我们评估了嗅球(OB)中的胃泌素受体(GHSRs)是否会增强嗅觉功能并影响觅食行为和新陈代谢:我们对嗅球GHSR缺失的小鼠(OBGHSR缺失)进行了详细的行为和代谢分析。我们还分析了OB scRNA-seq和空间转录组数据集,以评估主嗅球和附属嗅球以及前嗅核中的GHSR+细胞:结果:OBGHSR缺失影响了对食物和非食物气味的嗅觉辨别和习惯化。OBGHSR缺失后,焦虑样和抑郁样行为明显增加,而探索行为减少,在禁食条件下影响最大。OBGHSR缺失会影响进食行为,表现为进食次数和持续时间的改变,以及埋藏的寻食行为。OBGHSR 基因缺失会增加体重和脂肪量,降低脂肪利用率,并损害葡萄糖代谢,从而导致代谢功能障碍。OB scRNA-seq和空间转录组数据集的交叉引用分析显示,主嗅球和附属嗅球以及前嗅核中存在GHSR+谷氨酸神经元。消减嗅球中的谷氨酸神经元可减少胃泌素诱导的寻食行为,其结果与OBGHSR缺失后的表型相同:结论:OBGHSR 有助于维持嗅觉功能,尤其是在饥饿时,并促进行为适应,从而优化焦虑环境中的寻食行为,启动新陈代谢途径,为食物消耗做好准备。本文章由计算机程序翻译,如有差异,请以英文原文为准。
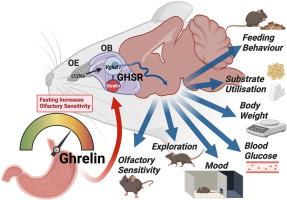
Hunger signalling in the olfactory bulb primes exploration, food-seeking and peripheral metabolism
Objective
Although the metabolic state of an organism affects olfactory function, the precise mechanisms and their impact on behavior and metabolism remain unknown. Here, we assess whether ghrelin receptors (GHSRs) in the olfactory bulb (OB) increase olfactory function and influence foraging behaviors and metabolism.
Methods
We performed a detailed behavioural and metabolic analysis in mice lacking GHSRs in the OB (OBGHSR deletion). We also analsyed OB scRNA-seq and spatial transcriptomic datasets to assess GHSR+ cells in the main and accessory olfactory bulbs, as well as the anterior olfactory nucleus.
Results
OBGHSR deletion affected olfactory discrimination and habituation to both food and non-food odors. Anxiety-like and depression-like behaviors were significantly greater after OBGHSR deletion, whereas exploratory behavior was reduced, with the greatest effect under fasted conditions. OBGHSR deletion impacted feeding behavior as evidenced by altered bout number and duration, as well as buried food-seeking. OBGHSR deletion increased body weight and fat mass, spared fat utilisation on a chow diet and impaired glucose metabolism indicating metabolic dysfunction. Cross referenced analysis of OB scRNA-seq and spatial transcriptomic datasets revealed GHSR+ glutamate neurons in the main and accessory olfactory bulbs, as well as the anterior olfactory nucleus. Ablation of glutamate neurons in the OB reduced ghrelin-induced food finding and phenocopied results seen after OBGHSR deletion.
Conclusions
OBGHSRs help to maintain olfactory function, particularly during hunger, and facilitate behavioral adaptations that optimise food-seeking in anxiogenic environments, priming metabolic pathways in preparation for food consumption.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
14.50
自引率
2.50%
发文量
219
审稿时长
43 days
期刊介绍:
Molecular Metabolism is a leading journal dedicated to sharing groundbreaking discoveries in the field of energy homeostasis and the underlying factors of metabolic disorders. These disorders include obesity, diabetes, cardiovascular disease, and cancer. Our journal focuses on publishing research driven by hypotheses and conducted to the highest standards, aiming to provide a mechanistic understanding of energy homeostasis-related behavior, physiology, and dysfunction.
We promote interdisciplinary science, covering a broad range of approaches from molecules to humans throughout the lifespan. Our goal is to contribute to transformative research in metabolism, which has the potential to revolutionize the field. By enabling progress in the prognosis, prevention, and ultimately the cure of metabolic disorders and their long-term complications, our journal seeks to better the future of health and well-being.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


