果冻制剂口服药物吸收的特征:膜渗透性和肠液容量的影响。
IF 3.7
3区 医学
Q2 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
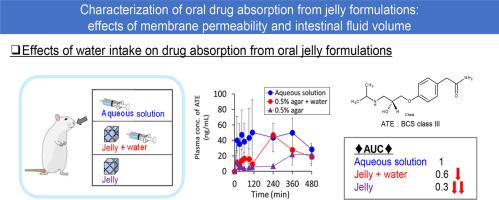
本研究旨在阐明果冻制剂的口服药物吸收过程。研究人员制备了含有不同膜渗透性药物(高:安替比林[ANT];中:美托洛尔[MET];低:阿替洛尔[ATE])的琼脂果冻和果胶果冻,并在大鼠体内进行了体外药物释放和体内药物吸收试验。除了果胶果冻在中性 pH 值下释放较快外,所有药物在两种果冻配方中的体外释放曲线相似。相比之下,大鼠体内对果冻制剂中 ATE 的吸收率明显低于对溶液中 ANT 的吸收率。口服后,琼脂胶冻对 ATE 和 MET 的吸收率较低,而额外摄入的水分可显著增加吸收率。药物吸收过程由果冻、肠液和血液组成的区室模型来描述。果冻中的药物扩散到肠液中,然后渗透到肠膜上。通过考虑限速过程,确定了琼脂果冻的药物吸收依赖于膜渗透性以及水摄入量的影响。总之,果冻制剂可能会减少和延迟药物的口服吸收,尤其是渗透性差的药物。肠液容量是控制药物吸收的重要因素之一。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Characterization of oral drug absorption from jelly formulations: Effects of membrane permeability and intestinal fluid volume
This study aims to clarify the process of oral drug absorption from jelly formulations. Agar and pectin-based jellies containing drugs with different membrane permeability (high: antipyrine [ANT], medium: metoprolol [MET], low: atenolol [ATE]) were prepared and tested for in vitro drug release and in vivo drug absorption in rats. All drugs showed similar release profiles in vitro from both jelly formulations, except for the faster release from pectin jelly at neutral pH. In contrast, in vivo absorption of ATE but not of ANT from jelly formulations was significantly lower than from solution. Absorption of ATE and MET was low from agar jelly after oral administration, whereas additional water intake significantly increased the absorption. The process of drug absorption was described by the compartmental model consisting of jelly, intestinal fluid, and blood compartments. Drugs in the jelly diffuse into the intestinal fluid and then permeate the intestinal membrane. By considering the rate-limiting process, membrane permeability-dependent drug absorption from agar jelly and the effects of water intake were identified. In conclusion, jelly formulations may potentially decrease and delay drug oral absorption, especially of poorly permeable drugs. Intestinal fluid volume is one of the important factors to control the drug absorption.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.30
自引率
13.20%
发文量
367
审稿时长
33 days
期刊介绍:
The Journal of Pharmaceutical Sciences will publish original research papers, original research notes, invited topical reviews (including Minireviews), and editorial commentary and news. The area of focus shall be concepts in basic pharmaceutical science and such topics as chemical processing of pharmaceuticals, including crystallization, lyophilization, chemical stability of drugs, pharmacokinetics, biopharmaceutics, pharmacodynamics, pro-drug developments, metabolic disposition of bioactive agents, dosage form design, protein-peptide chemistry and biotechnology specifically as these relate to pharmaceutical technology, and targeted drug delivery.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


