在异质结构铜铋催化剂上通过 CO2 和硝酸盐共电解合成尿素
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
将 CO2 和 NO3- 电催化偶联成尿素是一种很有前景的方法,可用于减少温室气体排放、减少工业过程中产生的废物以及储存可再生能源。然而,由于涉及多种反应物和反应的多步过程,其选择性和活性较差,限制了其应用。在此,我们首次报告了设计用于尿素电合成的异质结构铜铋双金属催化剂的工作。在 H 细胞中,尿素的法拉第效率(FE)高达 23.5%,生产率为 2180.3 μg h-1 mgcat-1,超过了文献中报道的大多数电催化剂。此外,该催化剂还具有显著的回收稳定性。实验和密度泛函理论计算表明,适量铋的引入诱导形成了具有丰富相界的铋-铜/O-铋/铜氧化物异质结构,这有利于 NO3-、CO2 和 H2O 的活化,并增强了 C-N 偶联,促进了 *HONCON 中间体的形成。此外,有利的 *HNCONH2 质子化和尿素解吸过程也得到了验证,进一步解释了对尿素具有高活性和选择性的原因。本文章由计算机程序翻译,如有差异,请以英文原文为准。
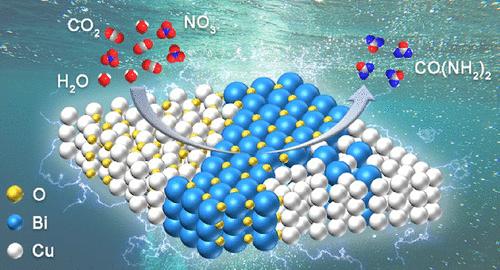
Urea Synthesis via Coelectrolysis of CO2 and Nitrate over Heterostructured Cu–Bi Catalysts
Electrocatalytic coupling of CO2 and NO3– to urea is a promising way to mitigate greenhouse gas emissions, reduce waste from industrial processes, and store renewable energy. However, the poor selectivity and activity limit its application due to the multistep process involving diverse reactants and reactions. Herein, we report the first work to design heterostructured Cu–Bi bimetallic catalysts for urea electrosynthesis. A high urea Faradaic efficiency (FE) of 23.5% with a production rate of 2180.3 μg h–1 mgcat–1 was achieved in H-cells, which surpassed most reported electrocatalysts in the literature. Moreover, the catalyst had a remarkable recycling stability. Experiments and density functional theory calculations demonstrated that introduction of moderate Bi induced the formation of the Bi–Cu/O–Bi/Cu2O heterostructure with abundant phase boundaries, which are beneficial for NO3–, CO2, and H2O activation and enhance C–N coupling and promote *HONCON intermediate formation. Moreover, favorable *HNCONH2 protonation and urea desorption processes were also validated, further explaining the reason for high activity and selectivity toward urea.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


