以亚洲人为主的FLT3突变急性髓性白血病复发/难治患者吉特替尼与挽救性化疗的3期研究
IF 12.8
1区 医学
Q1 HEMATOLOGY
引用次数: 0
摘要
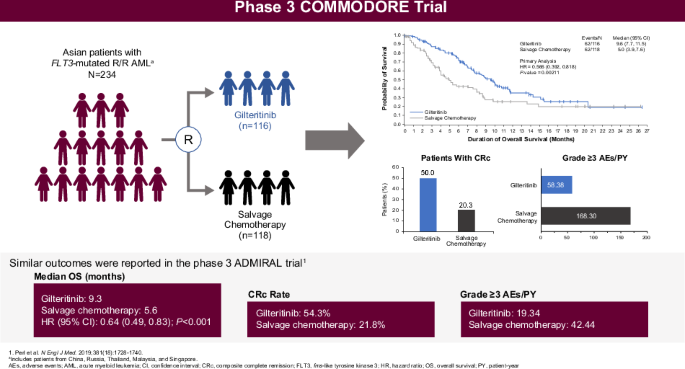
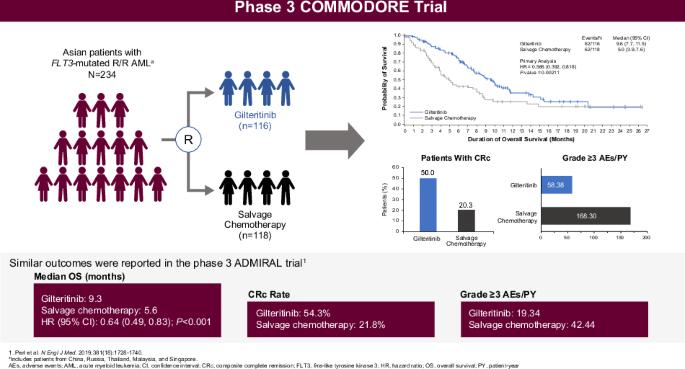
COMMODORE三期试验评估了吉特替尼与挽救性化疗(SC)在主要为亚洲人的复发/难治性(R/R)FLT3突变(FLT3mut+)急性髓性白血病(AML)患者群体中的疗效。主要终点是总生存期(OS);次要终点包括无事件生存期(EFS)和完全缓解率(CR)。截至2020年6月30日(中期分析:研究启动后32.2个月),234名患者被随机分组(吉瑞替尼,n = 116;SC,n = 118)。吉特替尼的中位OS明显长于SC(9.6个月对5.0个月;HR 0.566 [95% CI: 0.392, 0.818];p = 0.00211),中位随访时间为10.3个月。吉特替尼的中位 EFS 也明显更长(2.8 个月 vs. 0.6 个月;HR 0.551 [95% CI: 0.395, 0.769];p = 0.00004)。吉特替尼和SC的CR率分别为16.4%和10.2%;复合CR率分别为50.0%和20.3%。吉利替尼的暴露调整后≥3级不良事件(AE)发生率(58.38例/患者-年[E/PY])低于SC(168.30例/患者-年)。吉特替尼常见的AE为贫血(77.9%)和血小板减少(45.1%)。吉特替尼的血浆浓度在给药后约4小时达到峰值;多次给药会出现约3倍的蓄积。COMMODORE试验表明,吉特替尼能显著改善以亚洲患者为主的患者的OS和EFS,验证了吉特替尼在R/R FLT3mut+急性髓细胞白血病ADMIRAL试验中的疗效。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Phase 3 study of gilteritinib versus salvage chemotherapy in predominantly Asian patients with relapsed/refractory FLT3-mutated acute myeloid leukemia
The phase 3 COMMODORE trial evaluated gilteritinib versus salvage chemotherapy (SC) in a predominantly Asian relapsed/refractory (R/R) FLT3-mutated (FLT3mut+) acute myeloid leukemia (AML) patient population. The primary endpoint was overall survival (OS); secondary endpoints included event-free survival (EFS) and complete remission (CR) rate. As of June 30, 2020 (interim analysis: 32.2 months after study initiation), 234 patients were randomized (gilteritinib, n = 116; SC, n = 118). Median OS was significantly longer with gilteritinib versus SC (9.6 vs. 5.0 months; HR 0.566 [95% CI: 0.392, 0.818]; p = 0.00211) with a median follow-up of 10.3 months. Median EFS was also significantly longer with gilteritinib (2.8 vs. 0.6 months; HR 0.551 [95% CI: 0.395, 0.769]; p = 0.00004). CR rates with gilteritinib and SC were 16.4% and 10.2%, respectively; composite CR rates were 50.0% and 20.3%, respectively. Exposure-adjusted grade ≥3 adverse event (AE) rates were lower with gilteritinib (58.38 events/patient-year [E/PY]) versus SC (168.30 E/PY). Common AEs with gilteritinib were anemia (77.9%) and thrombocytopenia (45.1%). Gilteritinib plasma concentration peaked ~4 h postdose; ~3-fold accumulation occurred with multiple dosing. The COMMODORE trial demonstrated that gilteritinib significantly improved OS and EFS in predominantly Asian patients, validating the outcomes of gilteritinib from the ADMIRAL trial in R/R FLT3mut+ AML.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


