alpha-Gal C-二糖的合成
IF 3.3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
本文介绍了α-d-吡喃半乳糖基-(1 → 3)-d-吡喃半乳糖(α-Gal)的 C-二糖的合成,这是研究α-Gal 聚糖生物学的潜在工具。该合成策略以两种容易获得的前体 1,2-O-isopropylidene-d-glyceraldehyde 和 α-C-glactosyl-E-crotylboronate 反应为中心,可得到两种非对映异构的反rotylation 产物混合物。该反应的立体选择性由 (R)- 和 (S)-TRIP 催化剂控制,适当的非对映异构体被转化为 C-连接的 α-缬氨酰二糖,其中的苷元段包括 O-、C-和 S-糖苷实体,可用于糖醛酸合成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
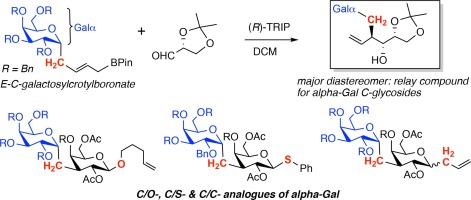
Synthesis of alpha-Gal C-disaccharides
The synthesis of C-disaccharides of α-d-galactopyranosyl-(1 → 3)-d-galactopyranose (α-Gal), potential tools for studying the biology of α-Gal glycans, is described. The synthetic strategy, centers on the reaction of two easily available precursors 1,2-O-isopropylidene-d-glyceraldehyde and an α-C-glactosyl-E-crotylboronate, which affords a mixture of two diastereomeric anti-crotylation products. The stereoselectivity of this reaction was controlled with (R)- and (S)-TRIP catalysts, and the appropriate diastereomer was transformed to C-linked disaccharides of α-Gal, in which the aglycone segment comprised O-, C- and S-glycoside entities that can enable glycoconjugate synthesis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


