手性布氏酸催化的吲哚与环丁酮通过级联 Friedel-Crafts/Semipinacol 重排的分子内不对称脱芳烃反应
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
通过吲哚与环丁酮的不对称脱芳烃反应,高效合成了融合有氮杂双环[2.2.1]庚酮分子的手性吲哚啉。合成了一种新的手性亚胺二磷酰亚胺(IDPi)催化剂,该催化剂在促进弗里德尔-卡夫斯/塞米那醇级联重排方面表现出非凡的活性。目标分子的制备收率高(高达 95%),对映体选择性好(高达 98%ee),操作方便。实验和计算研究相结合,为了解反应的能量分布和立体化学诱导提供了详细的机理。本文章由计算机程序翻译,如有差异,请以英文原文为准。
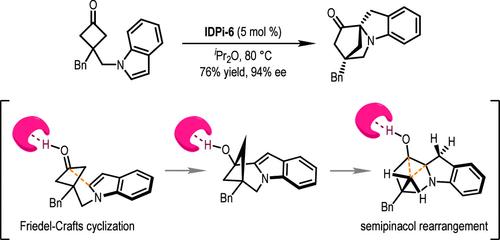
Chiral Brønsted Acid-Catalyzed Intramolecular Asymmetric Dearomatization Reaction of Indoles with Cyclobutanones via Cascade Friedel–Crafts/Semipinacol Rearrangement
The highly efficient synthesis of chiral indolines fused with an azabicyclo[2.2.1]heptanone moiety is achieved by an asymmetric dearomatization reaction of indoles with cyclobutanones. A new chiral imidodiphosphorimidate (IDPi) catalyst is synthesized and exhibits extraordinary activity in promoting a cascade Friedel–Crafts/semipinacol rearrangement. Target molecules are prepared in good yields (up to 95%) with excellent enantioselectivity (up to 98% ee) with operational convenience. Combined experimental and computational studies provide detailed mechanistic insights into the energy landscape and origin of the stereochemical induction of the reaction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


