通过预官能化的norcorroles的氧化扩环合成四种Ni(II)氮杂硼酸异构体
IF 4.7
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们探索了通过氧化插入预官能化的 Ni(II) norcorroles 来合成四种醛化和溴化偶氮硼烷的方法。我们还发现,Ni(II) 氮杂环戊烯与 Vilsmeier 试剂反应可生成 2-和 3-甲酰基氮杂环戊烯。我们通过高分辨率质谱、核磁共振和紫外-可见吸收光谱以及 X 射线衍射分析,对所有产物的结构进行了全面鉴定。通过合成甲酰基和溴偶氮硼烷的四种区域异构体,可以详细研究取代基对官能团位置的影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
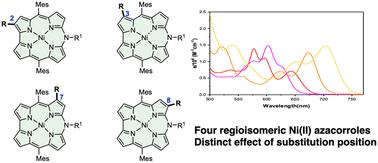
Synthesis of four regioisomeric Ni(ii) azacorroles via oxidative ring-expansion of prefunctionalised norcorroles†
We explored the synthesis of four regioisomeric formylated and brominated azacorroles via oxidative insertion of prefunctionalised Ni(II) norcorroles. We also found that the reaction of Ni(II) azacorroles with the Vilsmeier reagent afforded 2- and 3-formylazacorroles. The structures of all the products were characterised thoroughly by high-resolution mass spectrometry, NMR and UV-vis absorption spectroscopy together with X-ray diffraction analysis. The synthesis of four regioisomers of formyl- and bromoazacorroles allowed a detailed study of the substituent effect depending on the position of the functionality.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Chemistry Frontiers
CHEMISTRY, ORGANIC-
CiteScore
7.90
自引率
11.10%
发文量
686
审稿时长
1 months
期刊介绍:
Organic Chemistry Frontiers is an esteemed journal that publishes high-quality research across the field of organic chemistry. It places a significant emphasis on studies that contribute substantially to the field by introducing new or significantly improved protocols and methodologies. The journal covers a wide array of topics which include, but are not limited to, organic synthesis, the development of synthetic methodologies, catalysis, natural products, functional organic materials, supramolecular and macromolecular chemistry, as well as physical and computational organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


