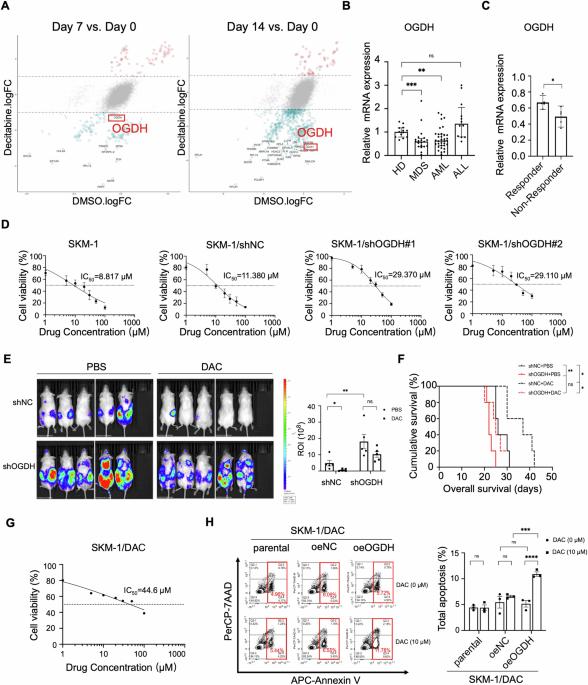
全基因组CRISPR/Cas9文库筛选发现OGDH是骨髓增生异常性肿瘤疾病进展和对地西他滨耐药性的调节因子,其作用是对谷氨酰胺代谢进行重编程
IF 12.8
1区 医学
Q1 HEMATOLOGY
引用次数: 0
摘要
骨髓增生异常肿瘤(MDS)是造血干细胞的克隆性疾病,以无效造血、全血细胞减少和发育不良为特征,通常会发展为急性髓性白血病(AML)[1]。地西他滨(DAC)是一种低甲基化药物(HMAs),在 MDS 的治疗中起着至关重要的作用 [2]。然而,HMAs 的完全缓解率仍然很低,仅为 15-20%,而且近一半的患者最终会在治疗过程中产生耐药性[3, 4]。MDS对地西他滨耐药的分子机制仍未完全明了[5]。随着对肿瘤发病机制认识的不断深入,代谢重编程,尤其是谷氨酰胺还原羧化的增强或氧化磷酸化的破坏,也被报道与血液恶性肿瘤的耐药性密切相关[6, 7]。在此,研究人员采用全基因组CRISPR/Cas9文库筛选和全外显子组测序(WES)相结合的方法,以鉴定涉及地西他滨敏感性的候选基因。OGDH编码三羧酸(TCA)循环中的一种关键酶,被确定为MDS对地西他滨耐药的调控因子。我们在体外和体内探讨了 OGDH 在调节谷氨酰胺代谢中的生物学功能。总之,我们首次报道了 OGDH 的低表达会增强 MDS 中谷氨酰胺的还原代谢。这些结果突显了 OGDH 在地西他滨耐药性中的重要作用,并为 MDS 的潜在治疗策略(包括靶向谷氨酰胺酶 (GLS))提供了启示。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Genome-wide CRISPR/Cas9 library screening identified OGDH as a regulator of disease progress and resistance to decitabine in myelodysplastic neoplasm by reprogramming glutamine metabolism
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


