无定形溶解度优势:理论思考、实验方法和当代意义。
IF 3.8
3区 医学
Q2 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
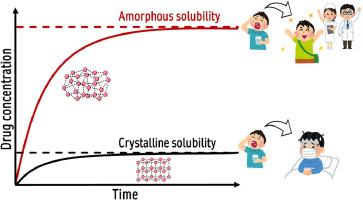
25 年前,汉考克和帕克斯提出了一个具有启发性的问题:"无定形药物的真正溶解度优势是什么?此后,由于理论认识和实验方法的重大进步,确定无定形溶解度的困难已被克服。现在,无定形溶解度被理解为药物经历液-液或液-玻璃相分离后的浓度,该分离形成了富含药物的水饱和相,与含有分子溶解药物的水相处于可移动的平衡状态。晶体溶解度是影响晶体药物制剂吸收的重要参数,而无定形溶解度则是考虑过饱和制剂吸收的重要因素。然而,药物的无定形溶解度非常复杂,尤其是在配方添加剂和胃肠道成分存在的情况下,基于浓度的测量可能无法显示药物的最大热力学活性。本综述将讨论无定形溶解度优势的概念,包括历史视角、理论考虑因素、无定形溶解度测量的实验方法,以及过饱和度和无定形溶解度对药物吸收的贡献。利用无定形溶解度并了解相关的物理化学原理,可以为水溶性差的药物制定更有效的开发策略,从而最终改善治疗效果。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Amorphous solubility advantage: Theoretical considerations, experimental methods, and contemporary relevance
Twenty-five years ago, Hancock and Parks asked a provocative question: “what is the true solubility advantage for amorphous pharmaceuticals?” Difficulties in determining the amorphous solubility have since been overcome due to significant advances in theoretical understanding and experimental methods. The amorphous solubility is now understood to be the concentration after the drug undergoes liquid-liquid or liquid-glass phase separation, forming a water-saturated drug-rich phase in metastable equilibrium with an aqueous phase containing molecularly dissolved drug. While crystalline solubility is an essential parameter impacting the absorption of crystalline drug formulations, amorphous solubility is a vital factor for considering absorption from supersaturating formulations. However, the amorphous solubility of drugs is complex, especially in the presence of formulation additives and gastrointestinal components, and concentration-based measurements may not indicate the maximum drug thermodynamic activity. This review discusses the concept of the amorphous solubility advantage, including a historical perspective, theoretical considerations, experimental methods for amorphous solubility measurement, and the contribution of supersaturation and amorphous solubility to drug absorption. Leveraging amorphous solubility and understanding the associated physicochemical principles can lead to more effective development strategies for poorly water-soluble drugs, ultimately benefiting therapeutic outcomes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.30
自引率
13.20%
发文量
367
审稿时长
33 days
期刊介绍:
The Journal of Pharmaceutical Sciences will publish original research papers, original research notes, invited topical reviews (including Minireviews), and editorial commentary and news. The area of focus shall be concepts in basic pharmaceutical science and such topics as chemical processing of pharmaceuticals, including crystallization, lyophilization, chemical stability of drugs, pharmacokinetics, biopharmaceutics, pharmacodynamics, pro-drug developments, metabolic disposition of bioactive agents, dosage form design, protein-peptide chemistry and biotechnology specifically as these relate to pharmaceutical technology, and targeted drug delivery.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


