瘤周组织 (PTT):越来越需要命名规范。
IF 6.4
1区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
有多种术语用于描述肿瘤附近的非恶性组织,这些组织属于肿瘤发生的器官。传统上,这些组织(有时称为 "正常邻近组织")被用作癌症研究的对照组,并被认为是形态健康的非癌组织的代表。然而,随着 OMIC 技术的发展,人们越来越认识到这些组织既不同于肿瘤组织,也不同于健康组织。此外,对这些组织的特性、特征以及在癌症形成和发展过程中的作用的研究也越来越多。为了使这一领域的未来研究更加协调、更容易获得,同时也为了消除人们对正常组织的普遍看法,我们主张有必要制定标准化的命名规则。为此,我们建议使用中性、全面的术语 "瘤周组织 "以及缩写词 "PTT"。虽然有关这些组织的大量数据已经公开,但由于缺乏样本采集程序的信息,这些数据的再利用仍然受到限制。为了便于今后重复使用这些数据,我们建议在样本采集过程中记录一系列特征。这些建议有助于标准操作程序的定义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
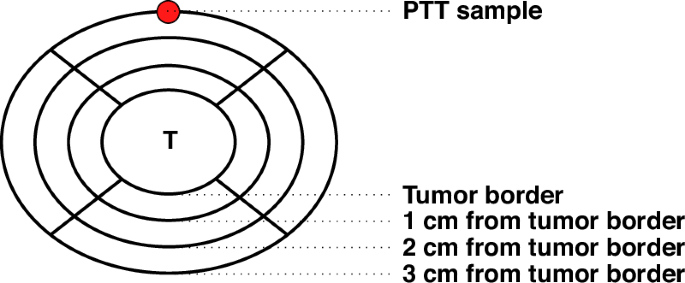
Peritumoral tissue (PTT): increasing need for naming convention
Various terms are used to describe non-malignant tissue located in the proximity of a tumor, belonging to the organ from which the tumor originated. Traditionally, these tissues, sometimes called “normal adjacent tissue” have been used as controls in cancer studies, and were considered representative of morphologically healthy, non-cancerous tissue. However, with the advancement of OMIC technologies, such tissues are increasingly recognized to be distinct from both tumor and healthy tissues. Furthermore, properties, characteristics, and role of these tissues in cancer formation and progression is increasingly studied. In order to make future research in this area more harmonized and more accessible, as well as to counter the widespread perception of normalcy, we are advocating the need for standardized naming convention. For this purpose, we propose the use of neutral and comprehensive term “Peritumoral Tissue” along with the acronym “PTT”. While significant amount of data on these tissues are publicly available, reuse of such data remains limited due to a lack of information on sample collection procedures. In order to facilitate future reuse of the data, we suggest a list of features that should be documented during sample collection procedures. These recommendations can aid the definition of Standard Operating Procedures.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

British Journal of Cancer
医学-肿瘤学
CiteScore
15.10
自引率
1.10%
发文量
383
审稿时长
6 months
期刊介绍:
The British Journal of Cancer is one of the most-cited general cancer journals, publishing significant advances in translational and clinical cancer research.It also publishes high-quality reviews and thought-provoking comment on all aspects of cancer prevention,diagnosis and treatment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


