空间转录组学揭示了小儿代谢功能障碍相关性脂肪性肝炎小鼠模型的转录组特征。
IF 4.7
2区 医学
Q1 PATHOLOGY
引用次数: 0
摘要
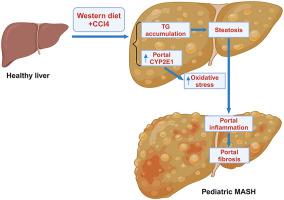
代谢功能障碍相关性脂肪性肝炎(MASH)被认为是代谢功能障碍相关性脂肪性肝病(MASLD)的进展形式,是儿童慢性肝病的主要病因。然而,由于缺乏动物模型,人们对小儿 MASH 的发病机制仍然知之甚少。在这项研究中,我们建立了小儿 MASH 的小鼠模型,并利用空间转录组学(ST)技术描述了肝脏转录组的特征。C57BL/6J 小鼠从 3 周大到 8 周大期间,在喂食西式饮食(WD)的同时每周注射四氯化碳(CCl4)。喂养5周后,WD+CCl4处理的小鼠出现了明显的肝损伤,但没有出现胰岛素抵抗。从组织学角度看,WD+CCl4诱导了2型MASH的主要特征,这是儿童中最常见的类型,其特征是肝脏脂肪变性、门静脉炎症和门静脉纤维化。通过对肝组织的 ST 分析,我们发现 WD+CCl4 组小鼠的第 0 簇富含与脂质代谢相关的通路。进一步研究发现,细胞色素 p450 2E1 (CYP2E1) 是第 0 组的首要标记基因,其在 WD+CCl4 组小鼠肝周围区域的表达增加。这些发现表明,我们的小儿MASH小鼠模型反映了人类MASH的组织学特征,而CYP2E1的上调可能与疾病的发病机制有关。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Spatial Transcriptomics Reveals the Transcriptomic Signatures in a Mouse Model of Pediatric Metabolic Dysfunction–Associated Steatohepatitis
Metabolic dysfunction–associated steatohepatitis (MASH) is considered the progressive form of metabolic dysfunction–associated steatotic liver disease, which is the leading cause of chronic liver disease in children. However, the pathogenesis of pediatric MASH remains poorly understood because of the lack of animal models. In this study, a mouse model of pediatric MASH was developed and its hepatic transcriptomic profile was characterized using spatial transcriptomics technology. C57BL/6J mice were fed a Western diet (WD) along with weekly injections of carbon tetrachloride (CCl4) from the age of 3 weeks and lasting up to 8 weeks. After 5 weeks of feeding, WD + CCl4–treated mice showed significant liver injury without the development of insulin resistance. Histologically, WD + CCl4 induced key features of type 2 MASH, the most common type observed in children, characterized by liver steatosis, portal inflammation, and portal fibrosis. Spatial transcriptomics analysis of liver tissues indicated that cluster 0 in the mouse from the WD + CCl4 group was enriched in pathways associated with lipid metabolism. Further investigation revealed that cytochrome p450 2E1 was the top marker gene of cluster 0, and its expression was increased in the periportal area of mice from the WD + CCl4 group. These findings suggest that this mouse model of pediatric MASH mirrors the histologic features of human MASH, and the up-regulation of cytochrome p450 2E1 may be linked to the disease pathogenesis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.40
自引率
0.00%
发文量
178
审稿时长
30 days
期刊介绍:
The American Journal of Pathology, official journal of the American Society for Investigative Pathology, published by Elsevier, Inc., seeks high-quality original research reports, reviews, and commentaries related to the molecular and cellular basis of disease. The editors will consider basic, translational, and clinical investigations that directly address mechanisms of pathogenesis or provide a foundation for future mechanistic inquiries. Examples of such foundational investigations include data mining, identification of biomarkers, molecular pathology, and discovery research. Foundational studies that incorporate deep learning and artificial intelligence are also welcome. High priority is given to studies of human disease and relevant experimental models using molecular, cellular, and organismal approaches.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


