基于线粒体转移的疗法可降低利氏综合征的发病率和死亡率
IF 18.9
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
摘要
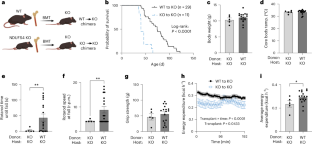
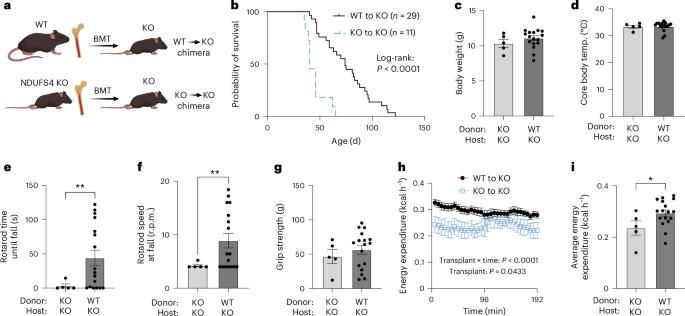
线粒体转移是最近描述的一种现象,即供体细胞向受体细胞输送线粒体1,2,3。线粒体转移的一个可能结果是为邻近细胞提供能量支持;例如,外源健康线粒体可挽救培养的ρ0细胞或Ndufs4-/-腹腔巨噬细胞线粒体代谢的细胞内在缺陷4,5,6,7。在自体造血干细胞移植前,让造血干细胞接触纯化的线粒体,可治疗大规模线粒体 DNA 变异患者的贫血症8,9,线粒体移植也被证明可最大限度地减少心脏10,11,12、大脑13,14,15 和四肢16 的缺血性损伤。然而,利用线粒体转移疗法治疗遗传性线粒体疾病的治疗潜力尚不清楚。在此,我们证明了 Ndufs4-/- Leigh 综合征(LS)小鼠模型在与线粒体转移相关的多种治疗模式中发病率和死亡率的改善。移植野生型小鼠的骨髓,可将造血细胞衍生的细胞外线粒体释放到血液循环中,并将线粒体转移到多个器官的宿主细胞中,从而改善小鼠的利氏综合征。此外,给野生型小鼠注射分离的线粒体可延长其寿命、改善神经功能并增加 Ndufs4-/- 小鼠的能量消耗,而 Ndufs4-/- 小鼠的线粒体不能改善神经功能。最后,我们证明给 Ndufs4-/- 小鼠施用跨物种人类线粒体也能改善 LS。这些数据表明,可以利用线粒体转移相关方法来治疗线粒体疾病,如 LS。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Mitochondria transfer-based therapies reduce the morbidity and mortality of Leigh syndrome
Mitochondria transfer is a recently described phenomenon in which donor cells deliver mitochondria to acceptor cells1–3. One possible consequence of mitochondria transfer is energetic support of neighbouring cells; for example, exogenous healthy mitochondria can rescue cell-intrinsic defects in mitochondrial metabolism in cultured ρ0 cells or Ndufs4−/− peritoneal macrophages4–7. Exposing haematopoietic stem cells to purified mitochondria before autologous haematopoietic stem cell transplantation allowed for treatment of anaemia in patients with large-scale mitochondrial DNA mutations8,9, and mitochondria transplantation was shown to minimize ischaemic damage to the heart10–12, brain13–15 and limbs16. However, the therapeutic potential of using mitochondria transfer-based therapies to treat inherited mitochondrial diseases is unclear. Here we demonstrate improved morbidity and mortality of the Ndufs4−/− mouse model of Leigh syndrome (LS) in multiple treatment paradigms associated with mitochondria transfer. Transplantation of bone marrow from wild-type mice, which is associated with release of haematopoietic cell-derived extracellular mitochondria into circulation and transfer of mitochondria to host cells in multiple organs, ameliorates LS in mice. Furthermore, administering isolated mitochondria from wild-type mice extends lifespan, improves neurological function and increases energy expenditure of Ndufs4−/− mice, whereas mitochondria from Ndufs4−/− mice did not improve neurological function. Finally, we demonstrate that cross-species administration of human mitochondria to Ndufs4−/− mice also improves LS. These data suggest that mitochondria transfer-related approaches can be harnessed to treat mitochondrial diseases, such as LS. Administration of exogenous mitochondria from mice or humans, or stimulation of mitochondria transfer from haematopoietic cells through bone marrow transplant from wild-type mice, is shown to improve morbidity and mortality in a mouse model of the mitochondrial disease Leigh syndrome.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


