ZNF460 介导的 APCDD1L-DT 上调通过抑制泛素介导的 DVL2 降解促进胆管癌的发展。
IF 4.8
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
摘要
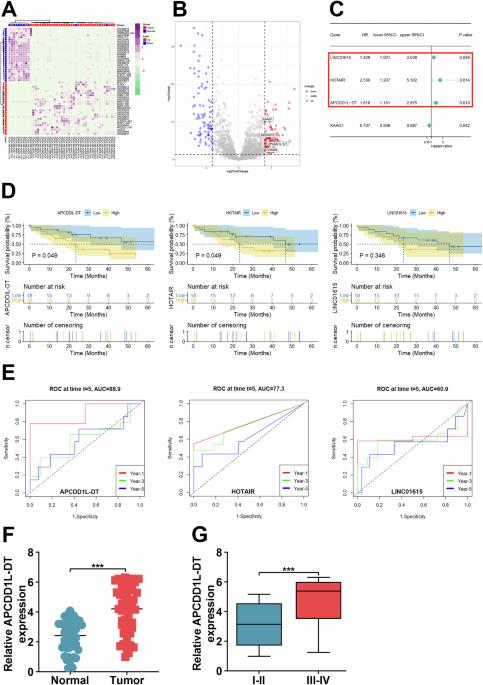
胆管癌(Colangiocarcinoma,CCA)以其侵袭性而闻名,在当前的医学领域,尤其是靶向疗法方面,它是一个巨大的挑战。在此背景下,长非编码 RNA(lncRNA)引起了研究人员的注意。这些独特的 RNA 被认为在各种癌症中发挥着关键作用,为开发更有效的治疗策略提供了前景广阔的途径。先前的研究证实了 APCDD1L-DT 在许多人类肿瘤中的异常表达,证明了它在疾病进展中的积极调控作用。尽管如此,APCDD1L-DT 在 CCA 中的生物学功能仍未完全明了。本研究首次记录了 APCDD1L-DT 在 CCA 标本中的异常表达,并将其与肿瘤患者的 TNM 分期密切联系起来。此外,抑制 APCDD1L-DT 的表达会阻碍肿瘤细胞的活力和运动。从机制上讲,转录因子 ZNF460 的异常激活正向调节了 APCDD1L-DT 在 CCA 中的表达。这种激活反过来又推动了 Wnt 通路的异常激活,通过阻碍泛素介导的 DVL2 降解来促进肿瘤的发展。总之,这项研究为理解 CCA 提供了良好的视角,并为应对这一令人生畏的恶性肿瘤提供了支持。本文章由计算机程序翻译,如有差异,请以英文原文为准。
ZNF460-mediated upregulation of APCDD1L-DT promotes cholangiocarcinoma development by inhibiting the ubiquitin-mediated degradation of DVL2
Cholangiocarcinoma (CCA), known for its aggressive nature, poses a formidable challenge in the current medical landscape, particularly in targeted therapies. Against this backdrop, long non-coding RNAs (lncRNAs) have captured the attention of researchers. These unique RNAs are believed to play pivotal roles in various cancers, offering promising avenues for the development of more effective treatment strategies. Previous studies have substantiated the aberrant expression of the APCDD1L-DT in numerous human tumors, demonstrating its positive regulatory roles in disease progression. Nevertheless, the biological functions of APCDD1L-DT in CCA are still not fully understood. This study marks the inaugural documentation of APCDD1L-DT exhibiting aberrant expression in CCA specimen, establishing a close correlation with the TNM staging of tumor patients. Furthermore, suppressing APCDD1L-DT expression hinders both the viability and motility of tumor cells. Mechanistically, the abnormal activation of the transcription factor ZNF460 positively regulated APCDD1L-DT expression in CCA. This activation, in turn, propels the abnormal activation of the Wnt pathway, fostering tumor development by impeding the ubiquitin-mediated degradation of DVL2. Broadly speaking, this study provides auspicious perspectives for comprehending CCA and furnishes support for addressing this daunting malignancy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


