抑制 IL-10R 可重塑肿瘤相关巨噬细胞并逆转多发性骨髓瘤的耐药性
IF 12.8
1区 医学
Q1 HEMATOLOGY
引用次数: 0
摘要
多发性骨髓瘤(MM)是骨髓中浆细胞的癌症,至今仍无法治愈。肿瘤微环境中的肿瘤相关巨噬细胞(TAMs)通常表现出亲肿瘤表型,并与肿瘤增殖、存活和耐药性相关。IL-10 是一种关键的免疫抑制细胞因子,可导致 TAMs 的招募和发展。在这项研究中,我们探讨了IL-10在MM TAM发展中的作用以及抑制IL-10/IL-10R/STAT3信号传导的治疗应用。我们证实,IL-10在MM骨髓中过表达,并在患者骨髓、体外三维共培养和小鼠模型中介导TAMs的M2样极化。反过来,TAMs 在体外和体内都会促进 MM 的增殖和耐药性。此外,使用阻断IL-10R单克隆抗体和STAT3蛋白降解剂/PROTAC抑制IL-10/IL-10R/STAT3轴,可防止TAMs的M2极化和TAM诱导的MM增殖,并在体外和体内使MM对治疗重新敏感。因此,我们的研究结果表明,抑制IL-10/IL-10R/STAT3轴是一种新型治疗策略,具有单药疗效,并可进一步与当前的抗MM疗法(如免疫调节药物)相结合,以克服耐药性。未来有必要对这种疗法在 MM 患者中的潜力进行评估。本文章由计算机程序翻译,如有差异,请以英文原文为准。
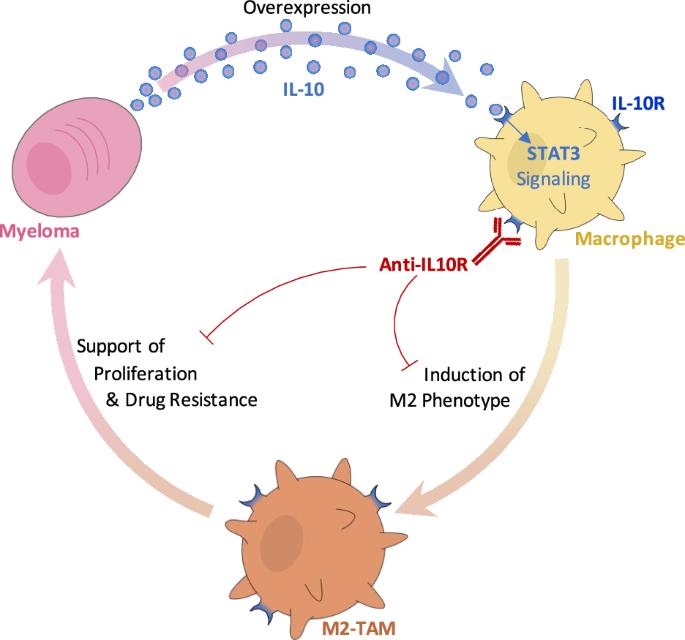
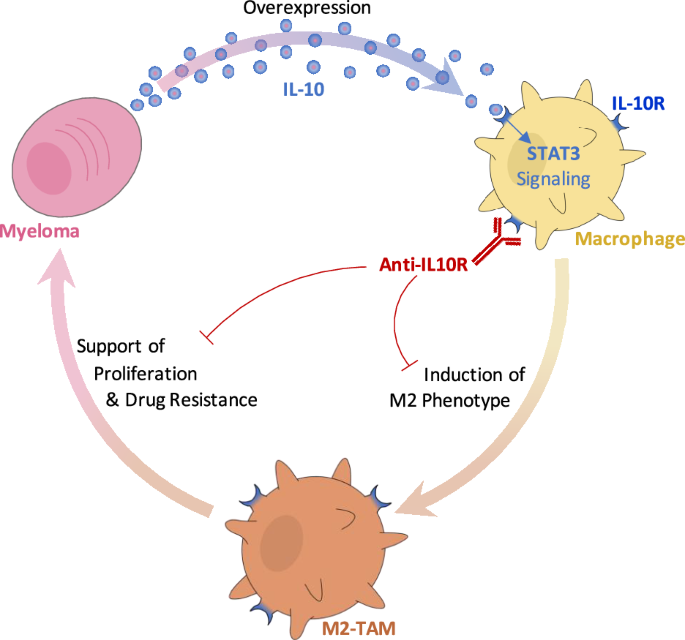
IL-10R inhibition reprograms tumor-associated macrophages and reverses drug resistance in multiple myeloma
Multiple myeloma (MM) is the cancer of plasma cells within the bone marrow and remains incurable. Tumor-associated macrophages (TAMs) within the tumor microenvironment often display a pro-tumor phenotype and correlate with tumor proliferation, survival, and therapy resistance. IL-10 is a key immunosuppressive cytokine that leads to recruitment and development of TAMs. In this study, we investigated the role of IL-10 in MM TAM development as well as the therapeutic application of IL-10/IL-10R/STAT3 signaling inhibition. We demonstrated that IL-10 is overexpressed in MM BM and mediates M2-like polarization of TAMs in patient BM, 3D co-cultures in vitro, and mouse models. In turn, TAMs promote MM proliferation and drug resistance, both in vitro and in vivo. Moreover, inhibition of IL-10/IL-10R/STAT3 axis using a blocking IL-10R monoclonal antibody and STAT3 protein degrader/PROTAC prevented M2 polarization of TAMs and the consequent TAM-induced proliferation of MM, and re-sensitized MM to therapy, in vitro and in vivo. Therefore, our findings suggest that inhibition of IL-10/IL-10R/STAT3 axis is a novel therapeutic strategy with monotherapy efficacy and can be further combined with current anti-MM therapy, such as immunomodulatory drugs, to overcome drug resistance. Future investigation is warranted to evaluate the potential of such therapy in MM patients.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


