改进、高效、简单的外消旋苯氧洛芬(一种非甾体抗炎药 (NSAID) )解析方法
IF 3
4区 化学
Q2 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
本文公开了一种用于制备非甾体抗炎药(NSAID)(S)-苯氧洛芬的改进、高效和简单的解析方法。外消旋苯氧洛芬的解析使用了一种易于获得、高效、可回收且成本低廉的手性试剂,即 (1R,2S)-(+)-顺式-1-氨基-2-茚满醇。这种新颖的解析工艺可获得纯度非常高的(S)-苯氧洛芬,其纯度大于 99%,而且基本上不含(R)-苯氧洛芬(小于 1%)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
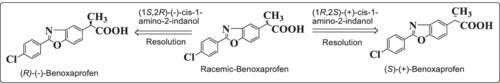
Improved, Efficient, and Simple Methodology for the Resolution of Racemic Benoxaprofen: A Nonsteroidal Anti-Inflammatory Drug (NSAID)
The present article discloses an improved, efficient, and simple resolution methodology for the preparation of (S)-benoxaprofen which is a nonsteroidal anti-inflammatory drug (NSAID). The resolution of racemic benoxaprofen uses an easily available, efficient, recoverable, and cost-effective chiral reagent, namely, (1R,2S)-(+)-cis-1-amino-2-indanol. This novel resolution process is having a very high purity of (S)-benoxaprofen, greater than 99%, substantially free from (R)-benoxaprofen (less than 1%).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
Chirality
医学-分析化学
CiteScore
4.40
自引率
5.00%
发文量
124
审稿时长
1 months
期刊介绍:
The main aim of the journal is to publish original contributions of scientific work on the role of chirality in chemistry and biochemistry in respect to biological, chemical, materials, pharmacological, spectroscopic and physical properties.
Papers on the chemistry (physiochemical, preparative synthetic, and analytical), physics, pharmacology, clinical pharmacology, toxicology, and other biological aspects of chiral molecules will be published.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


