脂肪酰基转移酶 1 的 m6A 修饰通过激活杯突酶抑制膀胱癌的进展。
IF 6.9
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
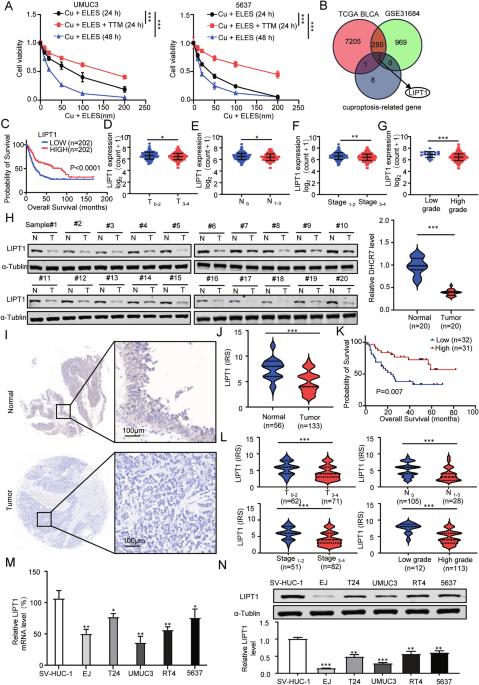
铜中毒是铜离子引起的细胞死亡过程,由与硫辛酸代谢有关的蛋白质脂化介导。铜中毒与各种肿瘤的进展和预后密切相关。在这里,我们发现脂酰基转移酶1(LIPT1)是一种与杯突症相关的关键基因,在膀胱癌(BLCA)中被下调,并与患者的不良预后相关。恢复 LIPT1 在膀胱癌细胞中的表达可抑制细胞增殖并促进杯突形成。此外,RNA测序和Bodipy染色的结果表明,LIPT1介导的代谢途径抑制了细胞中脂滴的积累,破坏了内质网(ER)的平衡,促进了细胞凋亡。此外,过表达 LIPT1 不仅会抑制 BLCA 细胞的体外增殖率,还会抑制其体内增殖率。从机理上讲,YTH N6-甲基腺苷 RNA 结合蛋白 F2(YTHDF2)以 m6A 依赖性方式促进了 LIPT1 mRNA 的降解。综上所述,这些结论揭示了LIPT1可促进杯状细胞变性和ER应激,从而抑制BLCA的进展,表明LIPT1将为治疗BLCA提供一个强有力的治疗方向和药物靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
m6A modification of lipoyltransferase 1 inhibits bladder cancer progression by activating cuproptosis
Cuproptosis, a cell death process caused by copper ions, is mediated by protein lipidation related to lipoic acid metabolism. There is a close connection between cuproptosis and the progression and prognosis of various tumors. Here, we identified lipoyltransferase 1 (LIPT1), a key gene related to cuproptosis, was downregulated in bladder cancer (BLCA) and was associated with unfavorable patient prognosis. Restoring the LIPT1 expression in BLCA cells suppressed the proliferation and promoted cuproptosis. Moreover, the consequences of RNA sequencing and Bodipy staining showed that the metabolic pathway mediated by LIPT1 inhibited the accumulation of lipid droplets in cells, disrupted endoplasmic reticulum (ER) homeostasis, and promoted cell apoptosis. Additionally, overexpression of LIPT1 not only repressed the proliferation rate of BLCA cells in vitro but also in vivo. Mechanistically, YTH N6-Methyladenosine RNA Binding Protein F2 (YTHDF2) promoted the degradation of LIPT1 mRNA in a m6A-dependent manner. In summary, these conclusions reveal that LIPT1 promotes cuprotosis and ER stress to inhibit the progression of BLCA, indicating that LIPT1 will provide a powerful treatment direction and drug target for treating BLCA.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


