十钨酸盐光催化偶氮嘧啶的 C(sp3)-H 自由基加成反应
IF 4.7
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本研究介绍了一种温和而高效的癸钨酸盐([W10O32]4-)光催化方法,该方法使用多种烷烃的一级、二级和三级非活性 C-H 键作为烷基化试剂,对偶氮嘧啶进行加氢烷基化反应。这种方法的显著特点包括:反应条件温和、原子和步骤效率高、适用底物范围广,以及能够促进复杂药物化合物的合成后定制。此外,该反应还可在连续流条件下有效放大到克级规模。本文章由计算机程序翻译,如有差异,请以英文原文为准。
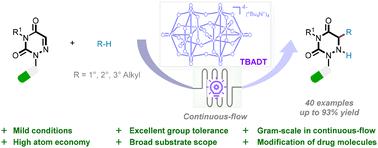
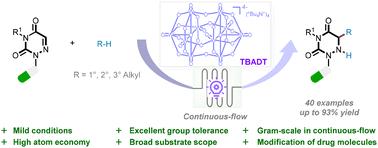
Decatungstate-photocatalyzed radical addition of C(sp3)–H to azauracils†
This study presents a gentle and remarkably effective decatungstate ([W10O32]4−)-photocatalyzed method for the hydroalkylation of azauracils using a wide range of primary, secondary, and tertiary unreactive C–H bonds of alkanes as the alkylating reagents. Notable features of this approach include its mild reaction conditions, significant atom and step efficiency, a broad range of applicable substrates, and its ability to facilitate the post-synthesis customization of complex pharmaceutical compounds. Additionally, the reaction could be effectively scaled up to a gram scale using continuous-flow conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Chemistry Frontiers
CHEMISTRY, ORGANIC-
CiteScore
7.90
自引率
11.10%
发文量
686
审稿时长
1 months
期刊介绍:
Organic Chemistry Frontiers is an esteemed journal that publishes high-quality research across the field of organic chemistry. It places a significant emphasis on studies that contribute substantially to the field by introducing new or significantly improved protocols and methodologies. The journal covers a wide array of topics which include, but are not limited to, organic synthesis, the development of synthetic methodologies, catalysis, natural products, functional organic materials, supramolecular and macromolecular chemistry, as well as physical and computational organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


