儿童 T 细胞急性淋巴细胞白血病中染色体三倍体的遗传特征和临床意义。
IF 12.8
1区 医学
Q1 HEMATOLOGY
引用次数: 0
摘要
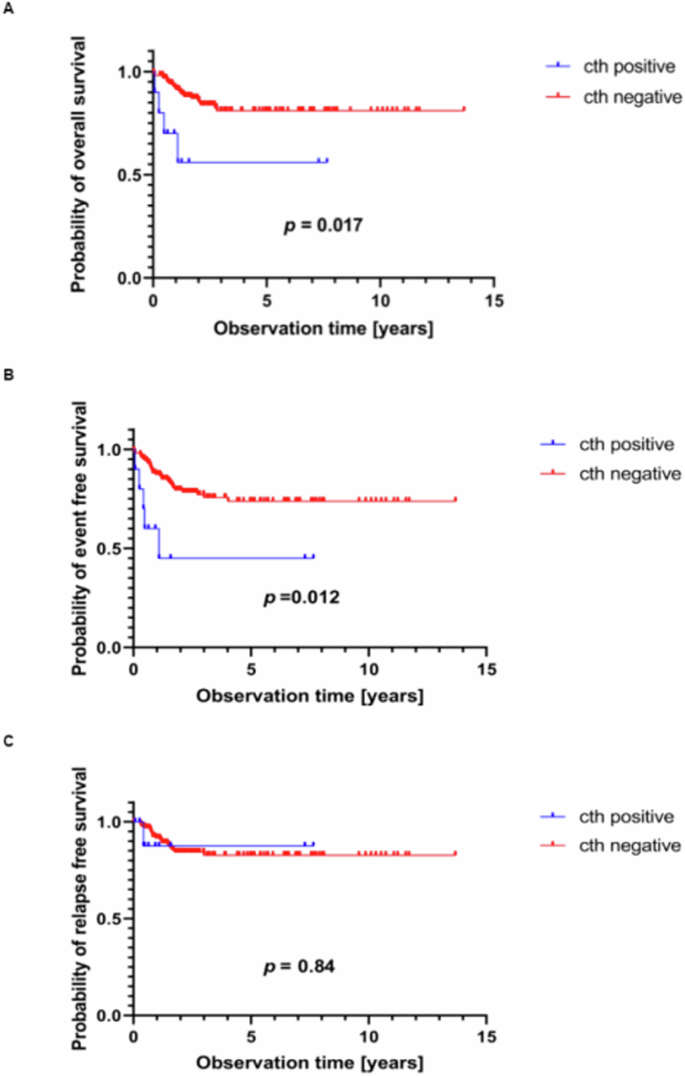
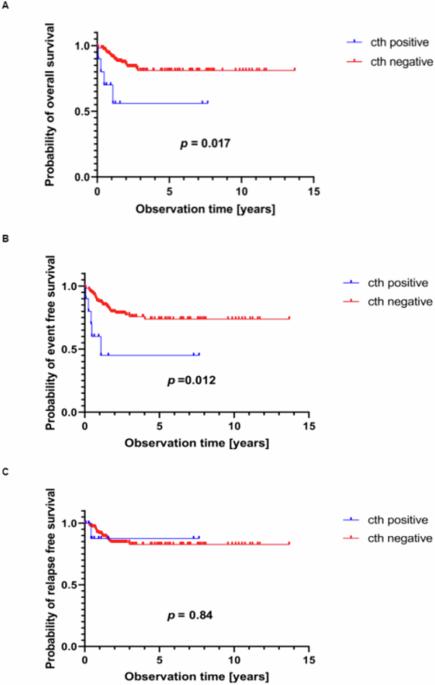
染色体重排(cth)是基因组不稳定的一种形式,在一次性的灾难性事件中导致大量新的染色体结构重排。它可导致促癌改变,如抑癌基因序列缺失、致癌融合的形成和癌基因扩增。我们研究了儿童 T 细胞急性淋巴细胞白血病(T-ALL)患者 cth 的遗传背景和临床意义。为此,我们使用高密度芯片分析了 173 名新确诊 T-ALL 儿童的全基因组拷贝数改变。在10个T-ALL样本(5.78%)中发现了Cth。其中6例样本的Cth发生在奈梅亨断裂综合征(5例)或李-弗劳米尼综合征(1例)的体质背景中。Cth产生的改变包括CDKN2A/B缺失(4例)和EZH2缺失(4例)、CDK6扩增(2例)以及NUP214::ABL1和TFG::GPR128融合。Cth阳性白血病表现出肿瘤抑制基因RB1(3例)、TP53(1例)和MED12(2例)的缺失。Cth阳性T-ALL患者的5年总生存率(OS)较低[0.56 vs. 0.81;危险比(HR)= 4.14 (1.42-12.02) p = 0.017],5年无事件生存率也较低[0.45 vs. 0.74;HR = 3.91 (1.52-10.08); p = 0.012]。在小儿T-ALL中,染色体三体是一种并不常见的基因组现象,但它与癌症易感综合征密切相关,并可能与预后不良有关。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Genetic hallmarks and clinical implications of chromothripsis in childhood T-cell acute lymphoblastic leukemia
Chromothripsis (cth) is a form of genomic instability leading to massive de novo structural chromosome rearrangements in a one-time catastrophic event. It can cause cancer-promoting alterations, such as loss of sequences for tumor-suppressor genes, formation of oncogenic fusions, and oncogene amplifications. We investigated the genetic background and clinical significance of cth in childhood T-cell acute lymphoblastic leukemia (T-ALL) patients. For this purpose, whole-genome copy number alterations were analyzed in 173 children with newly diagnosed T-ALL using high-density microarrays. Cth was identified in 10 T-ALL samples (5.78%). In six of them, cth occurred in a constitutional background of Nijmegen breakage syndrome (n = 5) or Li-Fraumeni syndrome (n = 1). Cth generated alterations, including deletions of CDKN2A/B (n = 4) and EZH2 (n = 4), amplifications of CDK6 (n = 2), and NUP214::ABL1 and TFG::GPR128 fusions. Cth-positive leukemias exhibited deletions involving the tumor-suppressor genes RB1 (n = 3), TP53 (n = 1) and MED12 (n = 2). Cth-positive T-ALL patients had a lower probability of 5-year overall survival (OS) [0.56 vs. 0.81; hazard ratio (HR) = 4.14 (1.42–12.02) p = 0.017] as did 5-year event-free survival [0.45 vs. 0.74; HR = 3.91 (1.52–10.08); p = 0.012]. Chromothripsis is an infrequent genomic phenomenon in pediatric T-ALL but is significantly associated with cancer-predisposing syndromes and may associate with inferior prognosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


