RBM12通过增加JAK1 mRNA的翻译来驱动肝细胞癌中PD-L1介导的免疫逃避。
IF 6.9
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
免疫抑制是 HCC 肿瘤微环境的特征,最近的研究表明 RNA 结合蛋白(RBPs)与 HCC 的发展有关。在此,我们进行了一项筛选,发现 RBM12 是一种能增加 PD-L1 表达的关键蛋白,从而推动了 HCC 的免疫逃避。此外,我们还发现 RBM12 在 HCC 组织中显著上调,并与 HCC 患者的不良预后相关。通过各种分子检测和高通量筛选,我们确定 RBM12 可通过其第 4-RRM(RNA 识别基序)结构域直接与 JAK1 mRNA 结合,并通过其第 2-RRM结构域招募 EIF4A2,从而增强核糖体在 JAK1 mRNA 上的分布,促进 JAK1 的翻译并随之上调其表达。因此,激活的 JAK1/STAT1 通路转录上调了 PD-L1 的表达,促进了 HCC 的免疫逃避。总之,我们的研究结果让人们深入了解了 RBM12 对 HCC 免疫逃避的重大贡献,并凸显了其作为未来治疗靶点的潜力。该图表摘要显示,RBM12在HCC中的高表达可通过提高JAK1 mRNA的翻译效率来增强PD-L1介导的肿瘤免疫逃避。本文章由计算机程序翻译,如有差异,请以英文原文为准。
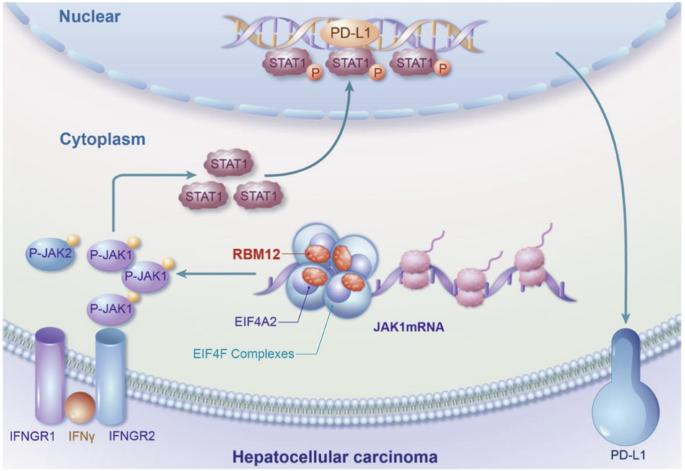
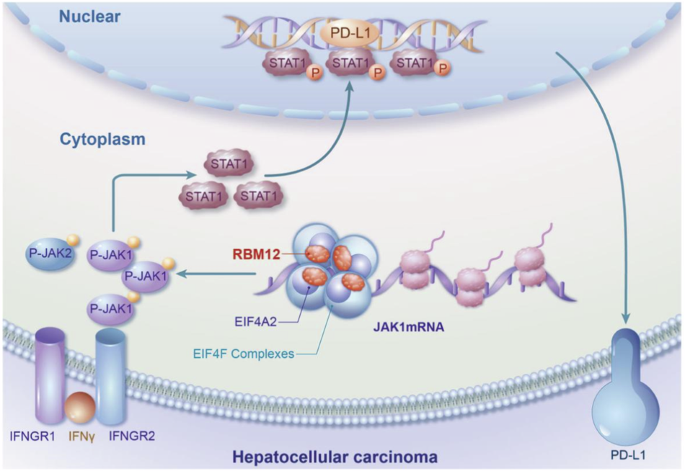
RBM12 drives PD-L1-mediated immune evasion in hepatocellular carcinoma by increasing JAK1 mRNA translation
Immunosuppression characterizes the tumour microenvironment in HCC, and recent studies have implicated RNA-binding proteins (RBPs) in the development of HCC. Here, we conducted a screen and identified RBM12 as a key protein that increased the expression of PD-L1, thereby driving immune evasion in HCC. Furthermore, RBM12 was found to be significantly upregulated in HCC tissues and was associated with a poor prognosis for HCC patients. Through various molecular assays and high-throughput screening, we determined that RBM12 could directly bind to the JAK1 mRNA via its 4th-RRM (RNA recognition motif) domain and recruit EIF4A2 through its 2nd-RRM domain, enhancing the distribution of ribosomes on JAK1 mRNA, which promotes the translation of JAK1 and the subsequent upregulation of its expression. As a result, the activated JAK1/STAT1 pathway transcriptionally upregulates PD-L1 expression, facilitating immune evasion in HCC. In summary, our findings provide insights into the significant contribution of RBM12 to immune evasion in HCC, highlighting its potential as a therapeutic target in the future.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


