James D. Robinson, Scott R. Sammons, Derek K. O'Flaherty
{"title":"制备用于研究非酶基因组复制的 2-Aminoimidazole-Activated Substrates。","authors":"James D. Robinson, Scott R. Sammons, Derek K. O'Flaherty","doi":"10.1002/cpz1.1119","DOIUrl":null,"url":null,"abstract":"<p>Nonenzymatic genome replication is thought to be an important process for primitive lifeforms, but this has yet to be demonstrated experimentally. Recent studies on the nonenzymatic primer extension mechanism mediated by nucleoside 5′-monophosphates (NMPs) activated with 2-aminoimidazole have revealed that imidazolium-bridged dinucleotide intermediates (N*N) account for the majority of the chemical copying process. As a result, an efficacious synthetic pathway for producing substrates activated with an imidazoyl moiety is desirable. This article provides a detailed protocol for the standard dehydrative redox reaction between NMPs and 2-aminoimidazole to produce nucleotide phosphoroimidazolides. In addition, we describe a similar synthetic pathway to produce N*N in high yields for homodimers. Finally, a simple reversed-phase cation exchange step is described to increase NMP solubility, which significantly increases yields for certain substrates. This approach allows for an efficient and cost-effective methodology to prepare high-quality substrates utilized in origins-of-life studies. © 2024 The Author(s). Current Protocols published by Wiley Periodicals LLC.</p><p><b>Basic Protocol 1</b>: Synthesis of 2-aminoimidazolephosphoroimidazolide-activated cytidine</p><p><b>Basic Protocol 2</b>: Synthesis of 2-aminoimidazolium-bridged dicytidyl intermediate</p><p><b>Basic Protocol 3</b>: Cation exchange of guanosine 5′-monophosphate disodium salt</p><p><b>Alternate Protocol</b>: Synthesis of cytidine 5′-phosphoroimidazolide or 2-aminoimidazolium-bridged dicytidyl from cytidine 5′-monophosphate disodium salt</p>","PeriodicalId":93970,"journal":{"name":"Current protocols","volume":"4 8","pages":""},"PeriodicalIF":0.0000,"publicationDate":"2024-08-26","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/cpz1.1119","citationCount":"0","resultStr":"{\"title\":\"Preparation of 2-Aminoimidazole-Activated Substrates for the Study of Nonenzymatic Genome Replication\",\"authors\":\"James D. Robinson, Scott R. Sammons, Derek K. O'Flaherty\",\"doi\":\"10.1002/cpz1.1119\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p>Nonenzymatic genome replication is thought to be an important process for primitive lifeforms, but this has yet to be demonstrated experimentally. Recent studies on the nonenzymatic primer extension mechanism mediated by nucleoside 5′-monophosphates (NMPs) activated with 2-aminoimidazole have revealed that imidazolium-bridged dinucleotide intermediates (N*N) account for the majority of the chemical copying process. As a result, an efficacious synthetic pathway for producing substrates activated with an imidazoyl moiety is desirable. This article provides a detailed protocol for the standard dehydrative redox reaction between NMPs and 2-aminoimidazole to produce nucleotide phosphoroimidazolides. In addition, we describe a similar synthetic pathway to produce N*N in high yields for homodimers. Finally, a simple reversed-phase cation exchange step is described to increase NMP solubility, which significantly increases yields for certain substrates. This approach allows for an efficient and cost-effective methodology to prepare high-quality substrates utilized in origins-of-life studies. © 2024 The Author(s). Current Protocols published by Wiley Periodicals LLC.</p><p><b>Basic Protocol 1</b>: Synthesis of 2-aminoimidazolephosphoroimidazolide-activated cytidine</p><p><b>Basic Protocol 2</b>: Synthesis of 2-aminoimidazolium-bridged dicytidyl intermediate</p><p><b>Basic Protocol 3</b>: Cation exchange of guanosine 5′-monophosphate disodium salt</p><p><b>Alternate Protocol</b>: Synthesis of cytidine 5′-phosphoroimidazolide or 2-aminoimidazolium-bridged dicytidyl from cytidine 5′-monophosphate disodium salt</p>\",\"PeriodicalId\":93970,\"journal\":{\"name\":\"Current protocols\",\"volume\":\"4 8\",\"pages\":\"\"},\"PeriodicalIF\":0.0000,\"publicationDate\":\"2024-08-26\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://onlinelibrary.wiley.com/doi/epdf/10.1002/cpz1.1119\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"Current protocols\",\"FirstCategoryId\":\"1085\",\"ListUrlMain\":\"https://onlinelibrary.wiley.com/doi/10.1002/cpz1.1119\",\"RegionNum\":0,\"RegionCategory\":null,\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"\",\"JCRName\":\"\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"Current protocols","FirstCategoryId":"1085","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/cpz1.1119","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"","JCRName":"","Score":null,"Total":0}
引用次数: 0
Preparation of 2-Aminoimidazole-Activated Substrates for the Study of Nonenzymatic Genome Replication
Nonenzymatic genome replication is thought to be an important process for primitive lifeforms, but this has yet to be demonstrated experimentally. Recent studies on the nonenzymatic primer extension mechanism mediated by nucleoside 5′-monophosphates (NMPs) activated with 2-aminoimidazole have revealed that imidazolium-bridged dinucleotide intermediates (N*N) account for the majority of the chemical copying process. As a result, an efficacious synthetic pathway for producing substrates activated with an imidazoyl moiety is desirable. This article provides a detailed protocol for the standard dehydrative redox reaction between NMPs and 2-aminoimidazole to produce nucleotide phosphoroimidazolides. In addition, we describe a similar synthetic pathway to produce N*N in high yields for homodimers. Finally, a simple reversed-phase cation exchange step is described to increase NMP solubility, which significantly increases yields for certain substrates. This approach allows for an efficient and cost-effective methodology to prepare high-quality substrates utilized in origins-of-life studies. © 2024 The Author(s). Current Protocols published by Wiley Periodicals LLC.
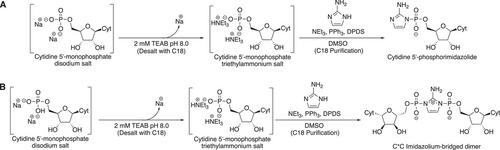
Basic Protocol 1: Synthesis of 2-aminoimidazolephosphoroimidazolide-activated cytidine
Basic Protocol 2: Synthesis of 2-aminoimidazolium-bridged dicytidyl intermediate
Basic Protocol 3: Cation exchange of guanosine 5′-monophosphate disodium salt
Alternate Protocol: Synthesis of cytidine 5′-phosphoroimidazolide or 2-aminoimidazolium-bridged dicytidyl from cytidine 5′-monophosphate disodium salt

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


