慢病毒载体基因疗法和 CFTR 调节剂在囊性纤维化大鼠气道模型中显示出相当的疗效。
IF 4.5
3区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
气道基因疗法等突变识别疗法有可能治疗任何囊性纤维化(CF)患者,无论其CF跨膜传导调节器(CFTR)基因变异情况如何。本研究的目的是利用两种CF大鼠模型(Phe508del和CFTR基因敲除(KO))来评估CFTR调节剂和慢病毒(LV)载体介导的基因疗法的比较效果。从大鼠气管中分离出细胞,用于建立气液界面(ALI)培养物。用调节剂组合 elexacaftor-tezacaftor-ivacaftor (ETI) 处理 Phe508del 大鼠 ALI,用 LV-CFTR 处理不同组的 Phe508del 和 KO 气管上皮细胞,然后在 ALI 上进行分化。进行乌星室测量以评估 CFTR 功能。经 ETI 处理的 Phe508del ALI 培养物的 CFTR 功能仅为野生型的 59%,而经基因添加疗法处理的 Phe508del 和 KO 细胞的 CFTR 功能分别恢复到野生型的 68% 和 47%。我们的研究结果表明,大鼠 Phe508del-CFTR 蛋白可通过 ETI 治疗成功挽救,而 CFTR 基因添加疗法可显著纠正 Phe508del 和 KO ALI 培养物中的 CFTR,使其达到与 ETI 相当的水平。这些发现凸显了基于 LV 载体的基因疗法治疗 CF 肺病的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
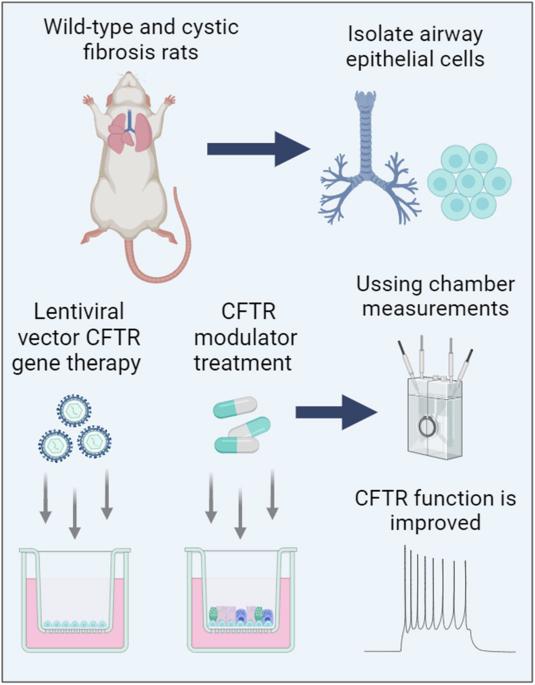
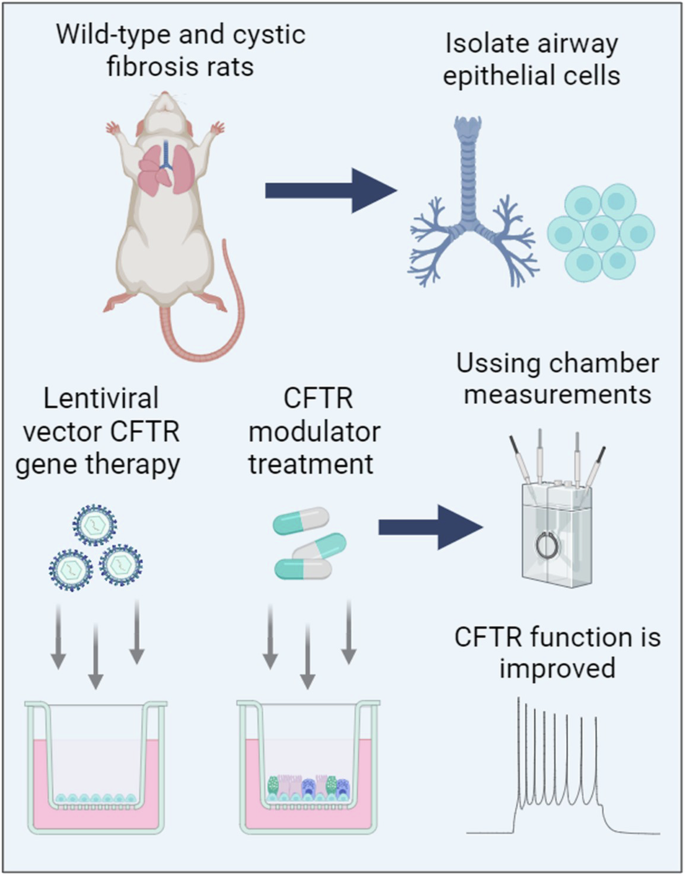
Lentiviral vector gene therapy and CFTR modulators show comparable effectiveness in cystic fibrosis rat airway models
Mutation-agnostic treatments such as airway gene therapy have the potential to treat any individual with cystic fibrosis (CF), irrespective of their CF transmembrane conductance regulator (CFTR) gene variants. The aim of this study was to employ two CF rat models, Phe508del and CFTR knockout (KO), to assess the comparative effectiveness of CFTR modulators and lentiviral (LV) vector-mediated gene therapy. Cells were isolated from the tracheas of rats and used to establish air-liquid interface (ALI) cultures. Phe508del rat ALIs were treated with the modulator combination, elexacaftor-tezacaftor-ivacaftor (ETI), and separate groups of Phe508del and KO tracheal epithelial cells were treated with LV-CFTR followed by differentiation at ALI. Ussing chamber measurements were performed to assess CFTR function. ETI-treated Phe508del ALI cultures demonstrated CFTR function that was 59% of wild-type level, while gene-addition therapy restored Phe508del to 68% and KO to 47% of wild-type level, respectively. Our findings show that rat Phe508del-CFTR protein can be successfully rescued with ETI treatment, and that CFTR gene-addition therapy provides significant CFTR correction in Phe508del and KO ALI cultures to levels that were comparable to ETI. These findings highlight the potential of an LV vector-based gene therapy for the treatment of CF lung disease.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Gene Therapy
医学-生化与分子生物学
CiteScore
9.70
自引率
2.00%
发文量
67
审稿时长
4-8 weeks
期刊介绍:
Gene Therapy covers both the research and clinical applications of novel therapeutic techniques based on a genetic component. Over the last few decades, significant advances in technologies ranging from identifying novel genetic targets that cause disease through to clinical studies, which show therapeutic benefit, have elevated this multidisciplinary field to the forefront of modern medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


