碘化物介导的膦 P(V/III)氧化还原偶联剂促成的醇类电化学氰化。
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们在此报告一种温和的电化学方法,利用市售试剂将醇类转化为相应的腈类。该方法可接受具有各种官能团(包括易氧化分解的官能团)的底物。机理研究揭示了一个关键的碘化物介导的膦电化学氧化途径,该途径导致烷氧基膦中间体,然后由亲氰亲核取代。这种方法证明了电化学在取代偶氮型试剂直接进行醇底物的亲核取代和同源反应中的应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
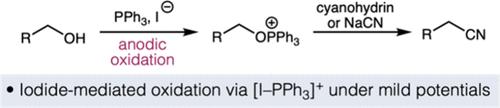
Electrochemical Cyanation of Alcohols Enabled by an Iodide-Mediated Phosphine P(V/III) Redox Couple.
We report herein a mild electrochemical method to transform alcohols into their corresponding nitriles by using commercially available reagents. This protocol accepts substrates with various functional groups including those that are susceptible to oxidative decomposition. Mechanistic studies revealed a critical iodide-mediated phosphine electrochemical oxidation pathway leading to the alkoxyphosphonium intermediate, followed by nucleophilic substitution by a cyanide nucleophile. This method demonstrates the use of electrochemistry in replacing azo-type reagents in direct nucleophilic substitution and homologation of alcohol substrates.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


