肝细胞特异性酪蛋白激酶 1 epsilon 消融可通过上调 TRAF3 改善小鼠代谢功能障碍相关性脂肪性肝炎。
IF 4.7
2区 医学
Q1 PATHOLOGY
引用次数: 0
摘要
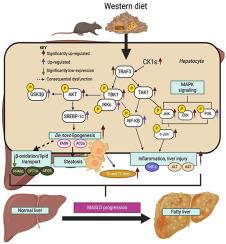
众所周知,酪蛋白激酶 1 epsilon(CK1ε)是丝氨酸/苏氨酸蛋白激酶家族的成员,能使多种底物磷酸化。然而,它在慢性肝病发展过程中的作用仍然难以捉摸。本研究旨在探讨 CK1ε 在代谢功能障碍相关性脂肪性肝炎(MASH)的发生和发展过程中的作用。通过将CK1ε等位基因缺失的小鼠(CK1εfl/fl)与Cre表达的Albumin小鼠杂交,产生肝细胞特异性CK1ε基因敲除(CK1εΔHEP)小鼠。用西式饮食(WD)或蛋氨酸和胆碱缺乏(MCD)喂养小鼠以诱导 MASH。CK1εΔHEP与WD或MCD诱导的MASH严重程度的降低有关,这可以从肝脏病变发生率的降低以及丙氨酸氨基转移酶、天门冬氨酸氨基转移酶和促炎细胞因子TNF-α水平的显著降低得到证实。CK1εΔHEP WD喂养小鼠的总胆固醇、甘油三酯和新生脂质基因均有明显改善,表明CK1ε可影响脂质代谢。CK1εΔHEP WD饲养小鼠的TRAF3、磷酸化(p)TAK1、pTBK1和pAKT水平明显下调,从而影响了下游MAPK信号转导,这表明了所观察到的拯救作用的潜在机制。最后,用 PF670462 对 CK1ε 进行药理抑制可改善 PA 诱导的体外脂肪性肝炎,并减轻 WD 诱导的体内代谢特征。总之,CK1ε会上调TRAF3,进而引起TAK1依赖性信号转导,放大下游MAPK信号转导,改变p-c-Jun水平,加剧炎症,所有这些都是WD诱导MASLD的因素。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Hepatocyte-Specific Casein Kinase 1 Epsilon Ablation Ameliorates Metabolic Dysfunction–Associated Steatohepatitis by Up-Regulating Tumor Necrosis Factor Receptor–Associated Factor 3 in Mice
Casein kinase 1 epsilon (CK1ε), a member of the serine/threonine protein kinase family, phosphorylates a broad range of substrates. However, its role in the development of chronic liver diseases remains elusive. This study aimed to investigate the role of CK1ε in the development and progression of metabolic dysfunction–associated steatohepatitis (MASH). Hepatocyte-specific CK1ε knockout (CK1εΔHEP) mice were generated by crossbreeding mice with floxed CK1ε alleles (CK1εfl/fl) and Cre-expressing albumin mice. Mice were fed either a Western diet (WD) or a methionine- and choline-deficient diet to induce MASH. CK1εΔHEP was associated with a decreased severity of WD- or methionine- and choline-deficient diet–induced MASH, as confirmed by reduced incidence of hepatic lesions and significantly lower levels of alanine aminotransferase, aspartate aminotransferase, and proinflammatory cytokine tumor necrosis factor (TNF)-α. CK1εΔHEP WD-fed mice exhibited significant amelioration of total cholesterol, triglycerides, and de novo lipogenic genes, indicating that CK1ε could influence lipid metabolism. CK1εΔHEP WD-fed mice showed significantly down-regulated TNF receptor–associated factor (TRAF) 3, phosphorylated (p) transforming growth factor-β–activated kinase 1, p–TRAF-associated NF-κB activator (TANK)-binding kinase 1 (TBK1), and p-AKT levels, thereby affecting downstream mitogen-activated protein kinase signaling, indicating a potential mechanism for the observed rescue. Finally, pharmacologic inhibition of CK1ε with PF670462 improved palmitic acid–induced steatohepatitis in vitro and attenuated WD-induced metabolic profile in vivo. In conclusion, CK1ε up-regulates TNF receptor–associated factor 3, which, in turn, causes transforming growth factor-β–activated kinase 1–dependent signaling, amplifies downstream mitogen-activated protein kinase signaling, modifies p-c-Jun levels, and exacerbates inflammation, all of which are factors in WD-induced metabolic dysfunction–associated steatotic liver disease.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.40
自引率
0.00%
发文量
178
审稿时长
30 days
期刊介绍:
The American Journal of Pathology, official journal of the American Society for Investigative Pathology, published by Elsevier, Inc., seeks high-quality original research reports, reviews, and commentaries related to the molecular and cellular basis of disease. The editors will consider basic, translational, and clinical investigations that directly address mechanisms of pathogenesis or provide a foundation for future mechanistic inquiries. Examples of such foundational investigations include data mining, identification of biomarkers, molecular pathology, and discovery research. Foundational studies that incorporate deep learning and artificial intelligence are also welcome. High priority is given to studies of human disease and relevant experimental models using molecular, cellular, and organismal approaches.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


