SIX1和DHX9之间的合作转录调节了KIRC中整合素-局灶粘附信号介导的转移和舒尼替尼耐药性。
IF 6.9
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
肾透明细胞癌(KIRC)细胞的高侵袭能力和获得性酪氨酸激酶抑制剂(TKI)耐药性仍然是延长晚期KIRC患者生存时间的障碍。在本研究中,我们发现在舒尼替尼耐药的KIRC细胞和转移性KIRC组织中,正弦眼同源框1(SIX1)被上调。随后,我们发现 SIX1 通过病灶粘附(Focal adhesion,FA)信号传导介导转移和舒尼替尼耐药,敲除 SIX1 可提高舒尼替尼在 KIRC 中的抗肿瘤效率。从机制上讲,FA 信号的上游基因 Integrin subunit beta 1 (ITGB1) 是 SIX1 的直接转录靶标。此外,我们还发现DexH-box螺旋酶9(DHX9)是SIX1诱导ITGB1转录的重要介质,沉默SIX1/DHX9复合物的亚基可显著降低ITGB1的转录。下调 SIX1 可减轻 DHX9 的核转位并减弱 DHX9 与 ITGB1 启动子的结合。总之,我们的研究结果揭示了 KIRC 中新的信号轴 SIX1/ITGB1/FAK,并为转移性 KIRC 患者找到了一种新的治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
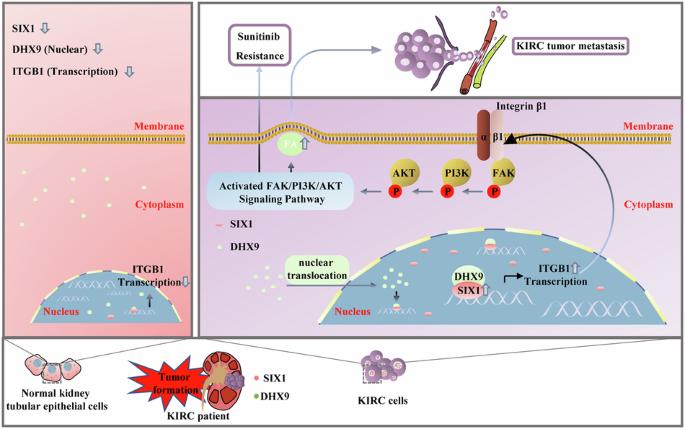
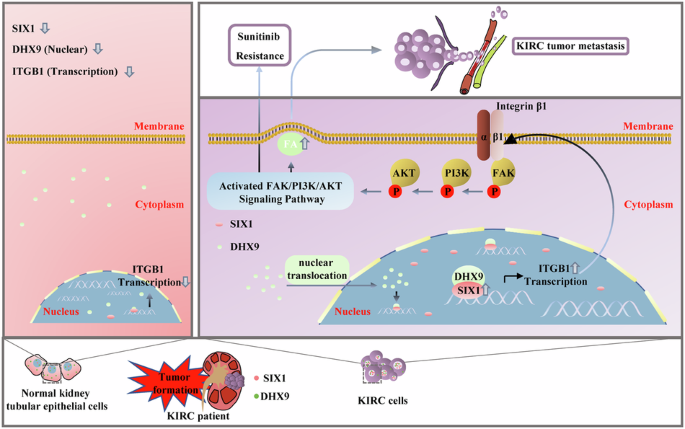
Cooperation between SIX1 and DHX9 transcriptionally regulates integrin-focal adhesion signaling mediated metastasis and sunitinib resistance in KIRC
High invasive capacity and acquired tyrosine kinase inhibitors (TKI) resistance of kidney renal clear cell carcinoma (KIRC) cells remain obstacles to prolonging the survival time of patients with advanced KIRC. In the present study, we reported that sine oculis homeobox 1 (SIX1) was upregulated in sunitinib-resistant KIRC cells and metastatic KIRC tissues. Subsequently, we found that SIX1 mediated metastasis and sunitinib resistance via Focal adhesion (FA) signaling, and knockdown of SIX1 enhanced the antitumor efficiency of sunitinib in KIRC. Mechanistically, Integrin subunit beta 1 (ITGB1), an upstream gene of FA signaling, was a direct transcriptional target of SIX1. In addition, we showed that DExH-box helicase 9 (DHX9) was an important mediator for SIX1-induced ITGB1 transcription, and silencing the subunits of SIX1/DHX9 complex significantly reduced transcription of ITGB1. Downregulation of SIX1 attenuated nuclear translocation of DHX9 and abrogated the binding of DHX9 to ITGB1 promoter. Collectively, our results unveiled a new signal axis SIX1/ITGB1/FAK in KIRC and identified a novel therapeutic strategy for metastatic KIRC patients.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


