有机引发剂引发的炔类化合物非定向 C-H 氨基化反应
IF 4.7
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在芳香族化合物的 C-H amination 反应中,对无过渡金属协议的需求非常大,但目前仍未实现。在本研究中,我们探讨了如何使用一种源自吡啶甲酸的有机引发剂来促进简单烷烃的非定向 C-H amination。通过对取代效应的研究,我们深入了解了电子和立体效应对 C-H amination 反应活性的影响。我们的方案在各种简单的炔类化合物和生物活性化合物的 C-H amination 反应中具有广泛的适用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
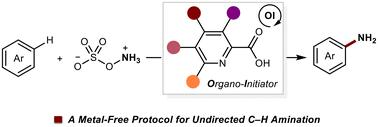
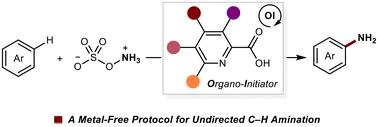
Organo-initiator enabled undirected C–H amination of arenes†
The demand for transition-metal-free protocols in the C–H amination of aromatic compounds is significant but remains elusive. In this study, we explore the use of a picolinic acid-derived organo-initiator to facilitate undirected C–H amination of simple arenes. An investigation of substitutional effects provides valuable insights into the electronic and steric influences on C–H amination reactivity. Our protocol exhibits broad applicability in the C–H amination of various simple arenes and bioactive compounds.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Chemistry Frontiers
CHEMISTRY, ORGANIC-
CiteScore
7.90
自引率
11.10%
发文量
686
审稿时长
1 months
期刊介绍:
Organic Chemistry Frontiers is an esteemed journal that publishes high-quality research across the field of organic chemistry. It places a significant emphasis on studies that contribute substantially to the field by introducing new or significantly improved protocols and methodologies. The journal covers a wide array of topics which include, but are not limited to, organic synthesis, the development of synthetic methodologies, catalysis, natural products, functional organic materials, supramolecular and macromolecular chemistry, as well as physical and computational organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


