绘制乳腺肿瘤微环境图:接近性分析揭示了巨噬细胞亚型与转移启动癌细胞之间的空间关系。
IF 6.9
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
转移是大多数癌症致死的原因。我们之前发现了导致转移的特定癌细胞群,在管腔型肿瘤中细胞角蛋白-14(CK14)和E-cadherin阳性,在三阴性肿瘤中E-cadherin和波形蛋白阳性。由于癌细胞是在由免疫细胞和基质细胞组成的复杂生态系统中进化的,我们试图破解这些侵袭性癌细胞群在肿瘤微环境(TME)中的空间相互作用。我们使用成像质谱仪检测了肿瘤芯片中的 36 种蛋白质,这些芯片包含来自管腔癌或三阴性乳腺癌(TNBC)的配对原发病灶和转移病灶,形成了一个包含 1,477,337 个注释细胞的数据集。我们重点研究了引发转移的细胞群,观察到它们与特定的成纤维细胞和巨噬细胞亚型非常接近,这种关系在原发性肿瘤和转移性肿瘤之间保持不变。值得注意的是,管腔癌细胞中的高 CK14 和 TNBC 细胞中的高波形蛋白与接近特定巨噬细胞亚型(CD163intCD206intPDL1intHLA-DR+或 PDL1highARG1high)相关。我们的深入空间分析表明,引发转移的癌细胞始终与TME内不同的细胞群聚集在一起,这表明这些细胞-细胞相互作用在促进转移方面发挥了作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
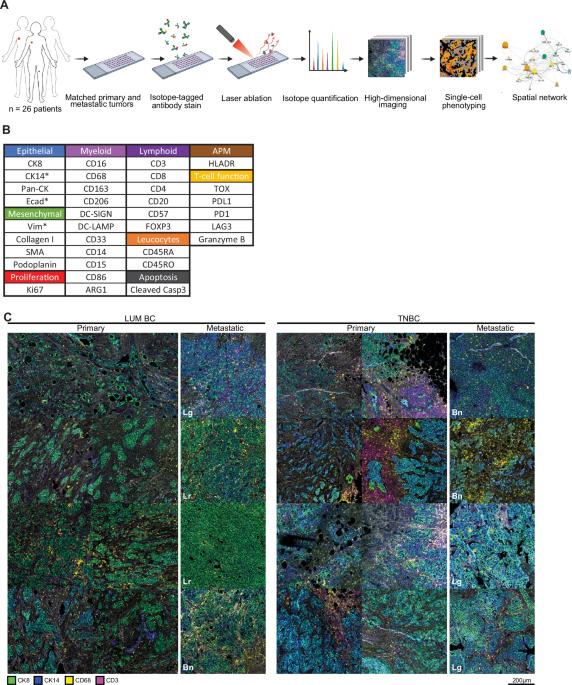
Mapping the breast tumor microenvironment: proximity analysis reveals spatial relationships between macrophage subtypes and metastasis-initiating cancer cells
Metastasis is responsible for the majority of cancer-related fatalities. We previously identified specific cancer cell populations responsible for metastatic events which are cytokeratin-14 (CK14) and E-cadherin positive in luminal tumors, and E-cadherin and vimentin positive in triple-negative tumors. Since cancer cells evolve within a complex ecosystem comprised of immune cells and stromal cells, we sought to decipher the spatial interactions of these aggressive cancer cell populations within the tumor microenvironment (TME). We used imaging mass cytometry to detect 36 proteins in tumor microarrays containing paired primary and metastatic lesions from luminal or triple-negative breast cancers (TNBC), resulting in a dataset of 1,477,337 annotated cells. Focusing on metastasis-initiating cell populations, we observed close proximity to specific fibroblast and macrophage subtypes, a relationship maintained between primary and metastatic tumors. Notably, high CK14 in luminal cancer cells and high vimentin in TNBC cells correlated with close proximity to specific macrophage subtypes (CD163intCD206intPDL1intHLA-DR+ or PDL1highARG1high). Our in-depth spatial analysis demonstrates that metastasis-initiating cancer cells consistently colocalizes with distinct cell populations within the TME, suggesting a role for these cell-cell interactions in promoting metastasis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


