Guo Chen, Wei Wang, Xin Wei, Yulin Chen, Liao Peng, Rui Qu, Yi Luo, Shengyin He, Yugao Liu, Jie Du, Ran Lu, Siying Li, Chuangwen Fan, Sujun Chen, Yi Dai, Luo Yang
下载PDF
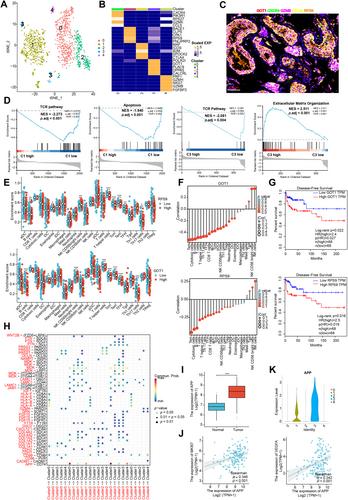
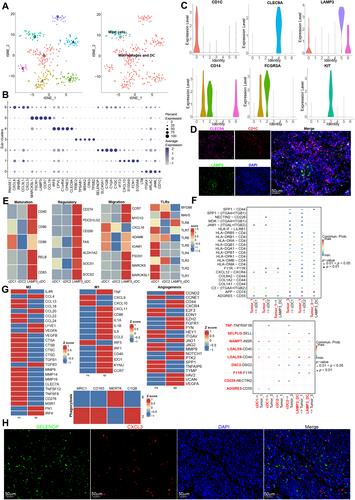
{"title":"单细胞转录组分析显示,APP-CD74 轴促进了免疫抑制和睾丸肿瘤的进展。","authors":"Guo Chen, Wei Wang, Xin Wei, Yulin Chen, Liao Peng, Rui Qu, Yi Luo, Shengyin He, Yugao Liu, Jie Du, Ran Lu, Siying Li, Chuangwen Fan, Sujun Chen, Yi Dai, Luo Yang","doi":"10.1002/path.6343","DOIUrl":null,"url":null,"abstract":"<p>Testicular tumors represent the most common malignancy among young men. Nevertheless, the pathogenesis and molecular underpinning of testicular tumors remain largely elusive. We aimed to delineate the intricate intra-tumoral heterogeneity and the network of intercellular communication within the tumor microenvironment. A total of 40,760 single-cell transcriptomes were analyzed, encompassing samples from six individuals with seminomas, two patients with mixed germ cell tumors, one patient with a Leydig cell tumor, and three healthy donors. Five distinct malignant subclusters were identified in the constructed landscape. Among them, malignant 1 and 3 subclusters were associated with a more immunosuppressive state and displayed worse disease-free survival. Further analysis identified that APP–CD74 interactions were significantly strengthened between malignant 1 and 3 subclusters and 14 types of immune subpopulations. In addition, we established an aberrant spermatogenesis trajectory and delineated the global gene alterations of somatic cells in seminoma testes. Sertoli cells were identified as the somatic cell type that differed the most from healthy donors to seminoma testes. Cellular communication between spermatogonial stem cells and Sertoli cells is disturbed in seminoma testes. Our study delineates the intra-tumoral heterogeneity and the tumor immune microenvironment in testicular tumors, offering novel insights for targeted therapy. © 2024 The Author(s). <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"264 3","pages":"250-269"},"PeriodicalIF":5.6000,"publicationDate":"2024-08-19","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6343","citationCount":"0","resultStr":"{\"title\":\"Single-cell transcriptomic analysis reveals that the APP–CD74 axis promotes immunosuppression and progression of testicular tumors\",\"authors\":\"Guo Chen, Wei Wang, Xin Wei, Yulin Chen, Liao Peng, Rui Qu, Yi Luo, Shengyin He, Yugao Liu, Jie Du, Ran Lu, Siying Li, Chuangwen Fan, Sujun Chen, Yi Dai, Luo Yang\",\"doi\":\"10.1002/path.6343\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p>Testicular tumors represent the most common malignancy among young men. Nevertheless, the pathogenesis and molecular underpinning of testicular tumors remain largely elusive. We aimed to delineate the intricate intra-tumoral heterogeneity and the network of intercellular communication within the tumor microenvironment. A total of 40,760 single-cell transcriptomes were analyzed, encompassing samples from six individuals with seminomas, two patients with mixed germ cell tumors, one patient with a Leydig cell tumor, and three healthy donors. Five distinct malignant subclusters were identified in the constructed landscape. Among them, malignant 1 and 3 subclusters were associated with a more immunosuppressive state and displayed worse disease-free survival. Further analysis identified that APP–CD74 interactions were significantly strengthened between malignant 1 and 3 subclusters and 14 types of immune subpopulations. In addition, we established an aberrant spermatogenesis trajectory and delineated the global gene alterations of somatic cells in seminoma testes. Sertoli cells were identified as the somatic cell type that differed the most from healthy donors to seminoma testes. Cellular communication between spermatogonial stem cells and Sertoli cells is disturbed in seminoma testes. Our study delineates the intra-tumoral heterogeneity and the tumor immune microenvironment in testicular tumors, offering novel insights for targeted therapy. © 2024 The Author(s). <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>\",\"PeriodicalId\":232,\"journal\":{\"name\":\"The Journal of Pathology\",\"volume\":\"264 3\",\"pages\":\"250-269\"},\"PeriodicalIF\":5.6000,\"publicationDate\":\"2024-08-19\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6343\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"The Journal of Pathology\",\"FirstCategoryId\":\"3\",\"ListUrlMain\":\"https://onlinelibrary.wiley.com/doi/10.1002/path.6343\",\"RegionNum\":2,\"RegionCategory\":\"医学\",\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"Q1\",\"JCRName\":\"ONCOLOGY\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6343","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


