lncRNA SIMALR的Ac4C修饰通过激活eEF1A2促进ITGB4/ITGA6的翻译,从而促进鼻咽癌的进展。
IF 6.9
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
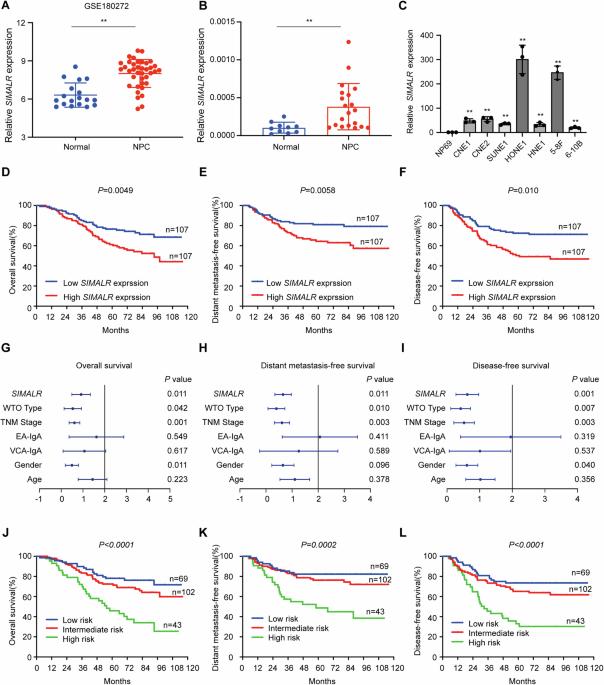
长非编码 RNA(lncRNA)的失调以多种方式参与调控肿瘤的进展。然而,人们对lncRNA是否参与蛋白质的翻译调控知之甚少。在此,我们通过分析 lncRNA 微阵列发现,炎性巨噬细胞凋亡抑制因子 lncRNA(SIMALR)在鼻咽癌组织中高表达。在临床上,SIMALR的高表达是鼻咽癌患者预后较差的独立预测因子。SIMALR是一种致癌lncRNA,可促进鼻咽癌细胞在体外和体内的增殖和转移。从机理上讲,SIMALR通过与真核细胞翻译机制中最关键的调节因子之一eEF1A2(真核翻译延伸因子1α2)结合,增强其内源性GTPase活性,从而成为蛋白质合成的关键加速器。此外,SIMALR介导了eEF1A2磷酸化的激活,从而加速了ITGB4/ITGA6的翻译,最终促进了鼻咽癌细胞的恶性表型。此外,N-乙酰转移酶10(NAT10)通过N4-乙酰胞苷(ac4C)修饰增强了SIMALR的稳定性,并导致其在鼻咽癌中过表达。总之,我们的研究结果说明了SIMALR作为蛋白质翻译加速器的功能,并强调了NAT10-SIMALR-eEF1A2-ITGB4/6轴在鼻咽癌中的致癌作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Ac4C modification of lncRNA SIMALR promotes nasopharyngeal carcinoma progression through activating eEF1A2 to facilitate ITGB4/ITGA6 translation
The dysregulation of long non-coding RNAs (lncRNAs) are involved in regulating tumor progression in multiple manner. However, little is known about whether lncRNA is involved in the translation regulation of proteins. Here, we identified that the suppressor of inflammatory macrophage apoptosis lncRNA (SIMALR) was highly expressed in nasopharyngeal carcinoma (NPC) tissues by analyzing the lncRNA microarray. Clinically, the high expression of SIMALR served as an independent predictor for inferior prognosis in NPC patients. SIMALR functioned as an oncogenic lncRNA that promoted the proliferation and metastasis of NPC cells in vitro and in vivo. Mechanistically, SIMALR served as a critical accelerator of protein synthesis by binding to eEF1A2 (eukaryotic translation elongation factor 1 alpha 2), one of the most crucial regulators in the translation machinery of the eukaryotic cells, and enhancing its endogenous GTPase activity. Furthermore, SIMALR mediated the activation of eEF1A2 phosphorylation to accelerate the translation of ITGB4/ITGA6, ultimately promoting the malignant phenotype of NPC cells. In addition, N-acetyltransferase 10 (NAT10) enhanced the stability of SIMALR and caused its overexpression in NPC through the N4-acetylcytidine (ac4C) modification. In sum, our results illustrate SIMALR functions as an accelerator for protein translation and highlight the oncogenic role of NAT10-SIMALR-eEF1A2-ITGB4/6 axis in NPC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


