强效抗疟药物单胞菌素衍生物立体化学库的合成及其立体化学修订。
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本研究介绍了一种具有苯并吡喃酮框架的强效抗疟先导化合物 2 的全合成及其立体化学构型的修正,该化合物来源于海洋真菌 Exserohilum sp.的 (+)-monocerin 天然产物。手性高价碘(III)催化的氧内酯化反应和后期的 O-甲基化反应是该合成的亮点,这使得我们能够获得 2 的所有可能的八种立体异构体库,从而进一步了解立体化学结构活性关系(S-SAR)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
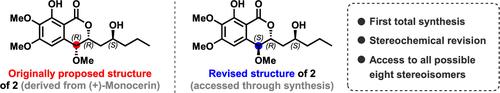
Synthesis of Stereochemical Library of a Potent Antimalarial Monocerin Derivative and Its Stereochemical Revision.
This study presents a total synthesis and revision of the stereochemical configuration of a potent antimalarial lead compound 2 possessing a benzo-pyranone framework, which was derived from the (+)-monocerin natural product of marine fungi, Exserohilum sp. Chiral hypervalent iodine(III)-catalyzed oxylactonization and late-stage O-methylation were highlights of the synthesis, which enabled access to the library of all possible eight stereoisomers of 2 for further understanding of stereochemical structure activity relationships (S-SARs).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


