长非编码 RNA EPCART 通过 PI3K/AKT/mTOR 通路和 PDCD4 调节前列腺癌的翻译。
IF 4.8
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
摘要
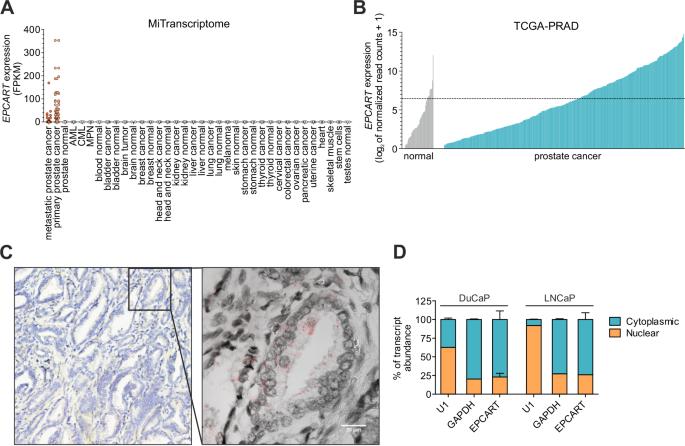
虽然已经发现了数百种与癌症相关的长非编码 RNA(lncRNA),但它们在癌细胞中的功能作用在很大程度上仍是一个谜。越来越多的lncRNA被认为在细胞质中发挥作用,例如作为翻译的调节因子。在这里,我们研究了 EPCART 的详细分子特征和功能作用,我们之前发现它是前列腺癌(PCa)中的潜在癌基因。首先,我们研究了EPCART的转录本结构,然后利用硅学方法证实EPCART是一种非肽链编码的lncRNA。对EPCART敲除细胞中差异表达的蛋白编码基因进行的通路分析表明,EPCART调节了PCa细胞的翻译机制。EPCART还主要位于细胞质和翻译位点。通过对EPCART基因敲除细胞进行定量蛋白质组分析,我们发现蛋白质翻译抑制剂PDCD4会因EPCART的减少而增加。进一步的研究表明,EPCART沉默对翻译的抑制作用是通过减少AKT的激活和抑制mTORC1通路介导的。综上所述,我们的研究结果表明,EPCART是一种与翻译相关的lncRNA,它通过调节PCa细胞中的PI3K/AKT/mTORC1通路发挥作用。此外,我们还提供了 PCa 肿瘤中 PDCD4 与 EPCART 相关的预后潜力证据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Long noncoding RNA EPCART regulates translation through PI3K/AKT/mTOR pathway and PDCD4 in prostate cancer
While hundreds of cancer-associated long noncoding RNAs (lncRNAs) have been discovered, their functional role in cancer cells is still largely a mystery. An increasing number of lncRNAs are recognized to function in the cytoplasm, e.g., as modulators of translation. Here, we investigated the detailed molecular identity and functional role of EPCART, a lncRNA we previously discovered to be a potential oncogene in prostate cancer (PCa). First, we interrogated the transcript structure of EPCART and then confirmed EPCART to be a non-peptide-coding lncRNA using in silico methods. Pathway analysis of differentially expressed protein-coding genes in EPCART knockout cells implied that EPCART modulates the translational machinery of PCa cells. EPCART was also largely located in the cytoplasm and at the sites of translation. With quantitative proteome analysis on EPCART knockout cells we discovered PDCD4, an inhibitor of protein translation, to be increased by EPCART reduction. Further studies indicated that the inhibitory effect of EPCART silencing on translation was mediated by reduced activation of AKT and inhibition of the mTORC1 pathway. Together, our findings identify EPCART as a translation-associated lncRNA that functions via modulation of the PI3K/AKT/mTORC1 pathway in PCa cells. Furthermore, we provide evidence for the prognostic potential of PDCD4 in PCa tumors in connection with EPCART.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


