可见光诱导光氧化催化的重氮甲基自由基组装点二/三官能化
IF 4.7
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
通过重氮甲基自由基的组装点官能化策略,开发了一种可见光诱导的光氧化催化重氮化合物与富含电子的壬烷或端烯烃的直接二/三官能化反应。该反应具有高区域选择性和广泛的底物范围,为一步合成α,α-二芳基和α,α,α-三芳基羰基化合物、α-芳基氨基酯、1,4-二烯和吲嗪类化合物提供了有效途径。对照实验和 DFT 计算表明,在光催化单电子转移过程中会形成游离的重氮甲基自由基中间体,从而为重氮化学的多样性开辟了一条新途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
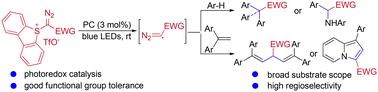
A visible light-induced photoredox-catalyzed assembly-point di/trifunctionalization of diazomethyl radicals†
A visible-light-induced photoredox-catalyzed direct di/trifunctionalization of diazo compounds with electron-rich arenes or terminal alkenes by an assembly-point functionalization strategy of diazomethyl radicals has been developed. This reaction exhibits high regioselectivity and a wide substrate scope and provides an efficient route toward the synthesis of α,α-diaryl- and α,α,α-triaryl-carbonyl compounds, α-arylamino esters, 1,4-dienes and indolizines in a single step. Control experiments and DFT calculations indicate that a free diazomethyl radical intermediate is formed via a photocatalytic single electron transfer process, opening up a novel means to access the diversity of diazo chemistry.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Chemistry Frontiers
CHEMISTRY, ORGANIC-
CiteScore
7.90
自引率
11.10%
发文量
686
审稿时长
1 months
期刊介绍:
Organic Chemistry Frontiers is an esteemed journal that publishes high-quality research across the field of organic chemistry. It places a significant emphasis on studies that contribute substantially to the field by introducing new or significantly improved protocols and methodologies. The journal covers a wide array of topics which include, but are not limited to, organic synthesis, the development of synthetic methodologies, catalysis, natural products, functional organic materials, supramolecular and macromolecular chemistry, as well as physical and computational organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


