LIFR 调节胆固醇驱动的肝细胞-中性粒细胞双向交流,促进肝脏再生
IF 18.9
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
摘要
肝脏再生受代谢和免疫调节的影响。尽管人们越来越认识到中性粒细胞参与了肝脏再生,但目前还不清楚肝脏在损伤后如何向骨髓发出释放中性粒细胞的信号,以及修复性中性粒细胞如何向肝细胞发出重新进入细胞周期的信号。在这里,我们报告了小鼠肝细胞中肝脏肿瘤抑制因子 Lifr 的缺失会损害肝脏,而白血病抑制因子受体(LIFR)的过表达则会促进肝部分切除术或毒性损伤后肝脏的修复和再生。肝脏受到物理或化学损伤时,肝细胞中的 LIFR 会以 STAT3 依赖性方式促进胆固醇和 CXCL1 的分泌,导致骨髓中性粒细胞外流到血液循环和受损肝脏。胆固醇通过其受体ERRα刺激中性粒细胞分泌肝细胞生长因子,从而加速肝细胞增殖。总之,我们的研究结果揭示了 LIFR-STAT3-CXCL1-CXCR2 轴和 LIFR-STAT3- 胆固醇-ERRα-肝细胞生长因子轴,它们形成了肝细胞-中性粒细胞的双向交叉对话,以修复和再生肝脏。本文章由计算机程序翻译,如有差异,请以英文原文为准。
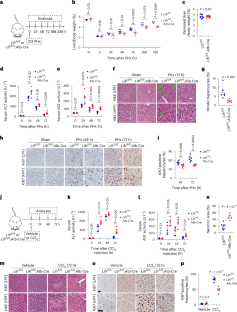
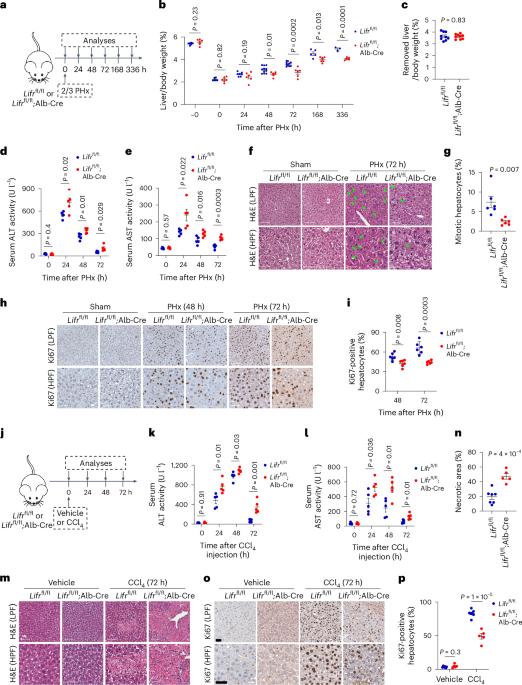
LIFR regulates cholesterol-driven bidirectional hepatocyte–neutrophil cross-talk to promote liver regeneration
Liver regeneration is under metabolic and immune regulation. Despite increasing recognition of the involvement of neutrophils in regeneration, it is unclear how the liver signals to the bone marrow to release neutrophils after injury and how reparative neutrophils signal to hepatocytes to reenter the cell cycle. Here we report that loss of the liver tumour suppressor Lifr in mouse hepatocytes impairs, whereas overexpression of leukaemia inhibitory factor receptor (LIFR) promotes liver repair and regeneration after partial hepatectomy or toxic injury. In response to physical or chemical damage to the liver, LIFR from hepatocytes promotes the secretion of cholesterol and CXCL1 in a STAT3-dependent manner, leading to the efflux of bone marrow neutrophils to the circulation and damaged liver. Cholesterol, via its receptor ERRα, stimulates neutrophils to secrete hepatocyte growth factor to accelerate hepatocyte proliferation. Altogether, our findings reveal a LIFR–STAT3–CXCL1–CXCR2 axis and a LIFR–STAT3–cholesterol–ERRα–hepatocyte growth factor axis that form bidirectional hepatocyte–neutrophil cross-talk to repair and regenerate the liver. The liver tumour suppressor LIFR plays a key role in liver repair and regeneration by orchestrating cholesterol-driven neutrophil hepatocyte growth factor production and hepatocyte–neutrophil cross-talk.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


