Isoxerophilusins A 和 B,来自 Isodon xerophilus 的两种新型多环不对称二萜二聚体:结构阐释、修饰和对α-葡萄糖苷酶的抑制活性
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
从 Isodon xerophilus 的根茎中分离出了 Isoxerophilusins A (1) 和 B (2),这是两种前所未有的二萜异二聚体,生物来源于 ent-atisanes 和 abietanes。通过大量光谱分析和单晶 X 射线衍射确定了它们的结构。1 的选择性酯化反应生成了 11 种新的衍生物。与阿卡波糖相比,所有衍生物都表现出了极佳的α-葡萄糖苷酶抑制活性。化合物 12 和 13 对α-葡萄糖苷酶有明显的抑制作用,其 IC50 值分别为 4.92 和 3.83 μM。本文章由计算机程序翻译,如有差异,请以英文原文为准。
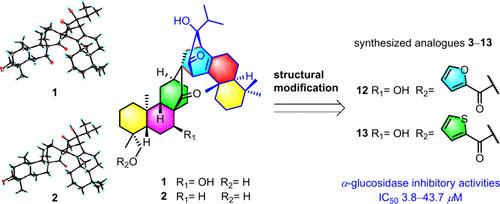
Isoxerophilusins A and B, Two Novel Polycyclic Asymmetric Diterpene Dimers from Isodon xerophilus: Structural Elucidation, Modification, and Inhibitory Activities against α-Glucosidase
Isoxerophilusins A (1) and B (2), two unprecedented diterpene heterodimers biogenetically from ent-atisanes and abietanes, were isolated from the rhizomes of Isodon xerophilus. Their structures were determined by extensive spectroscopic analysis and single-crystal X-ray diffraction. Selective esterification of 1 generated 11 new derivatives. All derivatives showed excellent α-glucosidase inhibitory activity in comparison to acarbose. Compounds 12 and 13 demonstrated significant inhibition against α-glucosidase with IC50 values of 4.92 and 3.83 μM, respectively.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


