3-oxo-24-nor-allobetulin 与费舍尔吲哚的区域特异性反应
IF 2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
首次观察到吲哚与三萜支架融合的特异性形成。从 3-oxo-24-nor-allobetulin 开始,费舍尔与苯肼在弱酸催化剂的作用下发生反应,生成了 3H-吲哚(indolenine)4。在这一过程中,C-4 甲基的构型发生了变化,X 射线衍射法证实了这一点。另一方面,3,2-吲哚 5 是在强酸催化剂的作用下合成的(使用 AcOH 酸中的盐酸苯肼或 MeOH 中的苯肼和几滴盐酸)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
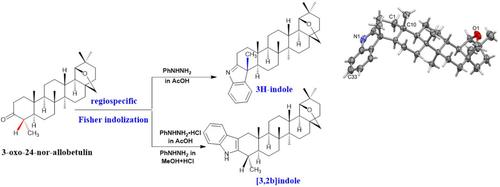
A regiospecific fischer indole reaction with 3-oxo-24-nor-allobetulin
First case of regiospecific formation of indole fused with a triterpene scaffold is observed. Starting from 3-oxo-24-nor-allobetulin Fischer reaction with phenylhydrazine under the influence of weak acid catalyst led to 3H-indole (indolenine) 4. This process was accompanied by a change in the configuration of the methyl group at C-4 that was confirmed by x-ray diffraction method. On the other hand, 3,2-indole 5 was synthesized under the influence of strong acid catalyst (using phenylhydrazine hydrochloride in AcOH acid or phenylhydrazine in MeOH with a few drops of HCl).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.20
自引率
4.20%
发文量
177
审稿时长
3.9 months
期刊介绍:
The Journal of Heterocyclic Chemistry is interested in publishing research on all aspects of heterocyclic chemistry, especially development and application of efficient synthetic methodologies and strategies for the synthesis of various heterocyclic compounds. In addition, Journal of Heterocyclic Chemistry promotes research in other areas that contribute to heterocyclic synthesis/application, such as synthesis design, reaction techniques, flow chemistry and continuous processing, multiphase catalysis, green chemistry, catalyst immobilization and recycling.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


