使用具有氧化还原活性的二(亚氨基)吡啶配体的 Ni(II) 复合物电催化亚硝酸盐还原成铵离子
IF 4.4
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
人类对氮循环的破坏促使人们探索亚硝酸盐的电催化还原。具有三叉氧化还原活性双(亚氨基)吡啶配体的均相 Ni(II) 复合物在 4-吗啉丙磺酸(MOPS)缓冲溶液中电催化将亚硝酸盐还原成铵离子和羟胺时,表现出很高的有效性和选择性。在 -1.4 V 对 Fc0/+ 的受控电位耦合作用下,主要产生铵离子,法拉第效率≥50%。波脚分析计算得出的翻转频率为 790 至 850 s-1。催化机理的计算研究深入揭示了拟议的化学步骤,并详细说明了电子和质子转移的能量学原理。本文章由计算机程序翻译,如有差异,请以英文原文为准。
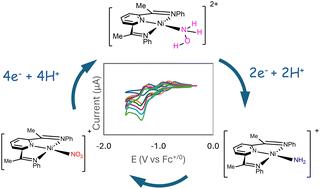
Electrocatalytic reduction of nitrite to ammonium ion using Ni(ii) complexes with redox-active di(imino)pyridine ligands†
Human disruption of the nitrogen cycle motivates the exploration into electrocatalytic reduction of nitrite. Homogeneous Ni(ii) complexes with tridentate redox-active bis(imino)pyridine ligands demonstrated high effectiveness and selectivity for electrocatalytic reduction of nitrite to the ammonium ion and hydroxylamine in solutions buffered with 4-morpholinepropanesulfonic acid (MOPS). Controlled potential coulometry at −1.4 V vs. Fc0/+ predominantly produced the ammonium ion with Faradaic efficiencies of ≥50%. Foot-of-the-wave analysis yielded calculated turn-over frequencies ranging from 790 to 850 s−1. Computational investigations of the catalytic mechanism provided insights into the proposed chemical steps and detailed the energetics of electron and proton transfers.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


