UGT1A7通过影响上皮细胞向间质转化改变了HER2阳性乳腺癌对曲妥珠单抗的反应:识别曲妥珠单抗耐药患者的潜在生物标记物。
IF 4.8
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
摘要
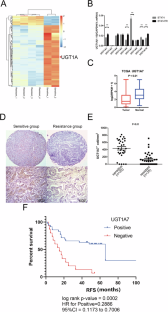
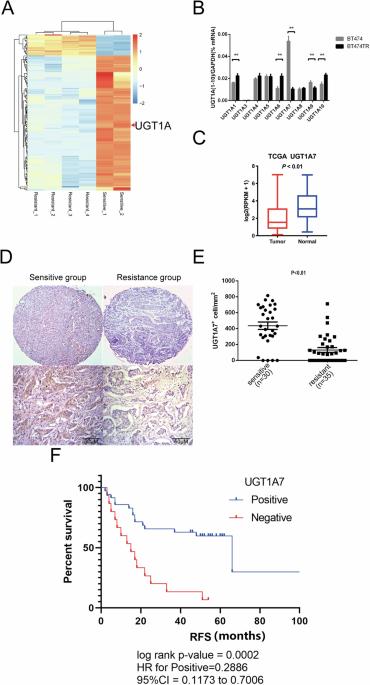
HER2阳性(HER2+)乳腺癌占所有乳腺癌的20%-30%。尽管曲妥珠单抗大大提高了HER2+乳腺癌患者的生存率,但70%以上的患者在治疗一年内会产生耐药性。我们对来自 GSE15043 的曲妥珠单抗敏感和耐药 HER2+ 乳腺癌细胞系进行了基因表达差异分析,以确定与曲妥珠单抗耐药相关的生物标记物。从曲妥珠单抗治疗敏感或耐药的临床 HER2+ 乳腺癌肿瘤样本中采集的 FFPE 组织中证实了生物标志物的差异表达。尿酸转移酶家族成员UGT1A7与曲妥珠单抗耐药性有关。在曲妥珠单抗耐药的肿瘤组织和产生曲妥珠单抗耐药的细胞系(BT474TR)中,UGT1A7的表达下调。在BT474TR中过表达UGT1A7可恢复它们对曲妥珠单抗治疗的敏感性,而在亲代细胞中下调UGT1A7的表达则会导致曲妥珠单抗耐药。重要的是,UGT1A7 定位于内质网并改变了应激反应。此外,通过影响 TWIST、SNAIL 和 GRP78 的表达以及 AMP 激活蛋白激酶信号通路,下调 UGT1A7 的表达会促进上皮细胞向间质转化(EMT),从而导致曲妥珠单抗耐药。这项研究证明了UGT1A7在肿瘤对曲妥珠单抗的反应中的重要作用和新机制。UGT1A7的低表达在EMT中起着重要作用,并导致曲妥珠单抗耐药。UGT1A7有可能被开发成一种生物标记物,用于识别对曲妥珠单抗治疗耐药的患者。本文章由计算机程序翻译,如有差异,请以英文原文为准。
UGT1A7 altered HER2-positive breast cancer response to trastuzumab by affecting epithelial-to-mesenchymal transition: A potential biomarker to identify patients resistant to trastuzumab treatment
HER2-positive (HER2+) breast cancer accounts for 20–30% of all breast cancers. Although trastuzumab has significantly improved the survival of patients with HER2+ breast cancer, more than 70% of patients develop drug resistance within one year of treatment. Differential-gene-expression analysis of trastuzumab-sensitive and resistant HER2+ breast cancer cell lines from GSE15043 was performed to identify the biomarkers associated with trastuzumab resistance. Differential biomarker expression was confirmed in FFPE tissues collected from clinical HER2+ breast cancer tumor samples that were sensitive or resistant to trastuzumab treatment. UGT1A7, a member of the uronic acid transferase family, was associated with trastuzumab resistance. UGT1A7 expression was downregulated in trastuzumab-resistant tumor tissues and in a cell line that developed trastuzumab resistance (BT474TR). Overexpressing UGT1A7 in BT474TR restored their sensitivity to trastuzumab treatment, whereas downregulating UGT1A7 expression in parental cells led to trastuzumab resistance. Importantly, UGT1A7 localized to the endoplasmic reticulum and altered stress responses. Furthermore, downregulating UGT1A7 expression promoted epithelial-to-mesenchymal transition (EMT) by affecting TWIST, SNAIL, and GRP78 expression and the AMP-activated protein kinase signaling pathway, thus contributing to trastuzumab resistance. This study demonstrated the important role and novel mechanisms of UGT1A7 in tumor responses to trastuzumab. Low UGT1A7 expression plays an important role in EMT and contributes to trastuzumab resistance. UGT1A7 has the potential to be developed as a biomarker for identifying patients who are resistant to trastuzumab treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


