可控合成新型镍钴氧化物-Co1-xCrxFe2O4(0 ≤ x ≤ 0.8)复合材料,用于碱性介质中的电解氧气生成和甲醇氧化
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
研究了尖晶石氧化物-尖晶石氧化物复合材料[NiCo2O4-Co1-xCrxFe2O4(0 ≤ x ≤ 0.8)]在氧进化反应(OER)和甲醇氧化反应(MOR)中的电化学性能。材料是通过共沉淀和溶胶-凝胶技术获得的。傅立叶变换红外光谱(FTIR)和 X 射线衍射(XRD)研究表明,存在近乎纯净的尖晶石相复合材料。通过 X 射线光电子能谱(XPS)评估了材料的价态分布。使用透射电子显微镜(TEM)测定了材料的粒度。使用扫描电子显微镜(SEM)对材料进行了形态分析。在 25 °C 的 KOH 溶液以及 KOH 和 CH3OH 溶液中进行了电化学研究,如循环伏安法(CV)和塔菲尔实验。阳极极化曲线用于确定材料的电催化性能,在 750 mV 的电位下,NiCo2O4-CoFe2O4 对 OER(j = 129.9 mAcm-2)和 MOR(j = 138.2 mAcm-2)的活性最高。通过计时器实验检验了电极的稳定性,并通过电化学阻抗谱(EIS)评估电化学活性表面积(ECSA)进一步了解了电催化活性的提高。通过捕捉 KOH 溶液以及 KOH 和 CH3OH 溶液中不同温度下的 Tafel 图,分析了标准电化学活化能 (ΔHel°#)、标准活化焓 (ΔH°#)和标准活化熵 (ΔS°#)等热力学参数。本文章由计算机程序翻译,如有差异,请以英文原文为准。
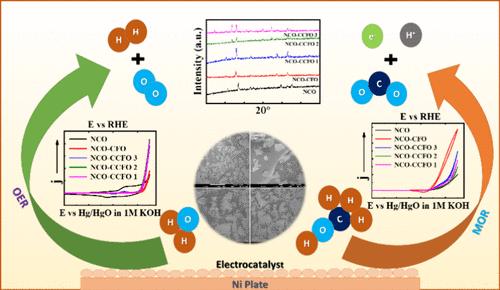
Controlled Synthesis of Novel NiCo2O4–Co1–xCrxFe2O4 (0 ≤ x ≤ 0.8) Composites for Electrolytic Oxygen Evolution and Methanol Oxidation in Alkaline Medium
The electrochemical performance of spinel oxide-spinel oxide composites [NiCo2O4–Co1–xCrxFe2O4 (0 ≤ x ≤ 0.8)] for the oxygen evolution reaction (OER) and methanol oxidation reaction (MOR) has been investigated. Materials were obtained by utilizing coprecipitation and sol–gel techniques. Fourier-transform infrared (FTIR) spectroscopy and X-ray diffraction (XRD) studies revealed the existence of nearly pure spinel phase composite materials. The valence distribution of the materials was assessed via X-ray photoelectron spectroscopy (XPS). The particle size of the materials was determined using transmission electron microscopy (TEM). The morphological analysis of the materials was performed using scanning electron microscopy (SEM). Electrochemical investigations like cyclic voltammetry (CV) and Tafel experiment were performed in KOH solution as well as KOH and CH3OH solution at 25 °C. Anodic polarization curves were used to determine the electrocatalytic performance of the materials and NiCo2O4–CoFe2O4 was observed to be most active toward the OER (j = 129.9 mAcm–2) and MOR (j = 138.2 mAcm–2) at a potential of 750 mV. The stability of the electrodes was examined via chronoamperometric experiments and further perception of the increment of electrocatalytic activity was acquired through the electrochemical active surface area (ECSA) assessments by means of electrochemical impedance spectroscopy (EIS). Thermodynamic parameters like standard electrochemical energy of activation (ΔHel°#), standard enthalpy of activation (ΔH°#), and standard entropy of activation (ΔS°#) were analyzed via capturing Tafel plot at various temperatures in KOH solution as well as KOH and CH3OH solution.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


