广谱抗生素会破坏平衡性渗出功能
IF 18.9
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
摘要
清除凋亡细胞,即所谓的细胞外排,对于组织平衡和预防自身免疫至关重要1。尽管过去的研究已经阐明了调控组织内同态渗出的局部分子信号2,3,但远端产生的信号是否也能调控同态渗出仍是一个未知数。在这里,我们发现在体内广谱抗生素(ABX)处理过的小鼠、万古霉素处理过的小鼠和无菌小鼠中,大腹腔巨噬细胞(LPM)的显示会损害流出细胞的功能。从机理上讲,微生物群衍生的短链脂肪酸丁酸盐能直接提高小鼠和人类巨噬细胞的出胞效率和能力,并能挽救 ABX 诱导的体内 LPM 出胞缺陷。对体外经丁酸盐处理的巨噬细胞进行大量信使 RNA 测序,以及对从经 ABX 处理和经丁酸盐挽救的小鼠体内分离出的 LPM 进行单细胞信使 RNA 测序,发现了支持渗出的转录程序的调控。具体而言,我们发现在经 ABX 处理的小鼠 LPMs 中,T 细胞免疫球蛋白和粘蛋白结构域包含 4(TIM-4,Timd4)的渗出受体下调,但口服丁酸盐可使其恢复正常。我们的研究表明,丁酸盐诱导的 LPM 渗出能力增强需要 TIM-4,而且在停用 ABX 后,LPM 的渗出能力会受到损害。在狼疮小鼠模型中,经 ABX 治疗的小鼠病情明显恶化。我们的研究结果表明,平衡性渗出依赖于远端代谢信号,并表明平衡性渗出缺陷可能解释了 ABX 的使用与炎症性疾病之间的联系4,5,6,7。本文章由计算机程序翻译,如有差异,请以英文原文为准。

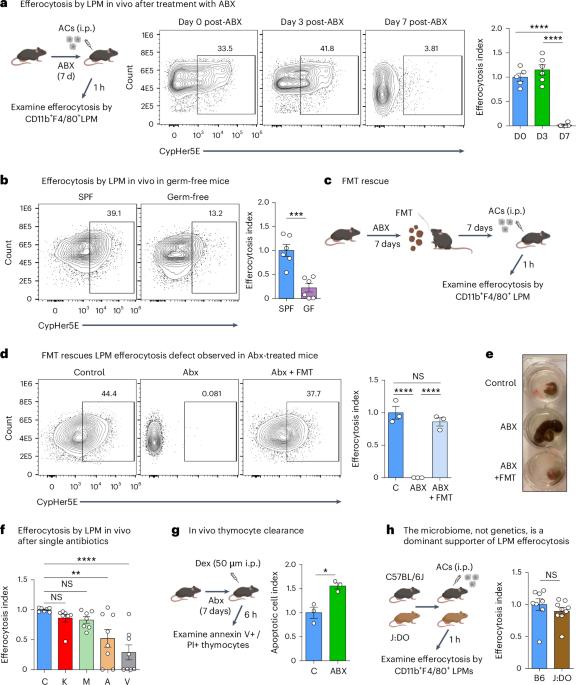
Broad-spectrum antibiotics disrupt homeostatic efferocytosis
The clearance of apoptotic cells, termed efferocytosis, is essential for tissue homeostasis and prevention of autoimmunity1. Although past studies have elucidated local molecular signals that regulate homeostatic efferocytosis in a tissue2,3, whether signals arising distally also regulate homeostatic efferocytosis remains elusive. Here, we show that large peritoneal macrophage (LPM) display impairs efferocytosis in broad-spectrum antibiotics (ABX)-treated, vancomycin-treated and germ-free mice in vivo, all of which have a depleted gut microbiota. Mechanistically, the microbiota-derived short-chain fatty acid butyrate directly boosts efferocytosis efficiency and capacity in mouse and human macrophages, and rescues ABX-induced LPM efferocytosis defects in vivo. Bulk messenger RNA sequencing of butyrate-treated macrophages in vitro and single-cell messenger RNA sequencing of LPMs isolated from ABX-treated and butyrate-rescued mice reveals regulation of efferocytosis-supportive transcriptional programmes. Specifically, we find that the efferocytosis receptor T cell immunoglobulin and mucin domain containing 4 (TIM-4, Timd4) is downregulated in LPMs of ABX-treated mice but rescued by oral butyrate. We show that TIM-4 is required for the butyrate-induced enhancement of LPM efferocytosis capacity and that LPM efferocytosis is impaired beyond withdrawal of ABX. ABX-treated mice exhibit significantly worse disease in a mouse model of lupus. Our results demonstrate that homeostatic efferocytosis relies on distal metabolic signals and suggest that defective homeostatic efferocytosis may explain the link between ABX use and inflammatory disease4–7. Saavedra et al. show that broad-spectrum antibiotics impair homeostatic efferocytosis of macrophages in mice by affecting the gut microbiota. Microbial butyrate is shown to rescue homeostatic efferocytosis efficiency in vivo.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


