GNAS 基因敲除可通过淋巴瘤中与病毒模仿相关的干扰素反应增强 HDAC3 抑制作用
IF 12.8
1区 医学
Q1 HEMATOLOGY
引用次数: 0
摘要
尽管选择性 HDAC3 抑制在 CREBBP 突变的淋巴瘤亚群中显示出前景,但野生型肿瘤通常表现出耐药性。在这里,我们利用无偏见的全基因组CRISPR筛选,确定了GNAS基因敲除(KO)是抗性淋巴瘤细胞对HDAC3抑制的增敏剂。从机理上讲,GNAS KO诱导的增敏作用与典型的G蛋白活性无关,而是意外地由病毒模拟相关的干扰素(IFN)反应介导,其特征是TBK1和IRF3活化、双链RNA形成和转座元件(TE)表达。GNAS KO 还能与 HDAC3 抑制协同增强 CD8+ T 细胞诱导的细胞毒性。此外,我们在人类淋巴瘤患者中观察到,GNAS 的低表达与基线 TE 的高表达和 IFN 信号的上调有关,并且与 GNAS KO 在组蛋白修饰、mRNA 处理和转录调控方面具有共同的生物活性。总之,我们的研究结果在淋巴瘤的 HDAC3 抑制和病毒模仿之间建立了前所未有的联系。我们建议将 GNAS 的低表达作为一种潜在的生物标志物,它反映了在淋巴瘤(尤其是 CREBBP 野生型病例)的临床治疗中,病毒模仿会增强对 HDAC3 抑制的反应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
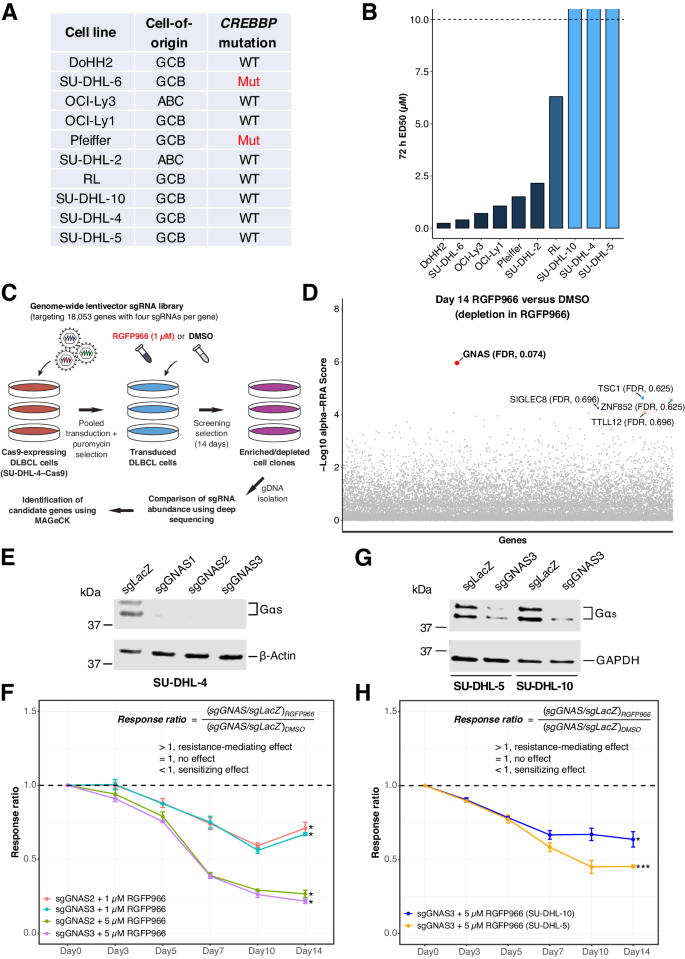
GNAS knockout potentiates HDAC3 inhibition through viral mimicry-related interferon responses in lymphoma
Despite selective HDAC3 inhibition showing promise in a subset of lymphomas with CREBBP mutations, wild-type tumors generally exhibit resistance. Here, using unbiased genome-wide CRISPR screening, we identify GNAS knockout (KO) as a sensitizer of resistant lymphoma cells to HDAC3 inhibition. Mechanistically, GNAS KO-induced sensitization is independent of the canonical G-protein activities but unexpectedly mediated by viral mimicry-related interferon (IFN) responses, characterized by TBK1 and IRF3 activation, double-stranded RNA formation, and transposable element (TE) expression. GNAS KO additionally synergizes with HDAC3 inhibition to enhance CD8+ T cell-induced cytotoxicity. Moreover, we observe in human lymphoma patients that low GNAS expression is associated with high baseline TE expression and upregulated IFN signaling and shares common disrupted biological activities with GNAS KO in histone modification, mRNA processing, and transcriptional regulation. Collectively, our findings establish an unprecedented link between HDAC3 inhibition and viral mimicry in lymphoma. We suggest low GNAS expression as a potential biomarker that reflects viral mimicry priming for enhanced response to HDAC3 inhibition in the clinical treatment of lymphoma, especially the CREBBP wild-type cases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


