EZH2 PROTACs 可靶向 EZH2- 和 FOXM1 相关致癌节点,抑制乳腺癌细胞生长。
IF 6.9
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
乳腺癌(BC)仍然是女性癌症相关死亡的第二大原因。雌激素受体(ER)抑制剂他莫昔芬等激素疗法的抗药性是治疗乳腺癌的一大障碍。多聚核抑制复合体 2(PRC2)的甲基转移酶成分泽斯特同源物增强子 2(EZH2)与他莫昔芬的抗药性有关。有证据表明,EZH2 通常以一种非规范的、不依赖于甲基转移酶的方式,通过与致癌转录因子相互作用而发挥转录辅激活剂的功能。与甲基转移酶抑制剂不同,蛋白水解靶向嵌合体(PROTAC)可以抑制 EZH2 的激活和抑制功能。在这里,我们发现与甲基转移酶抑制剂相比,EZH2 PROTACs(MS177和MS8815)能更有效地抑制包括他莫昔芬耐药在内的BC细胞的生长。从机理上讲,EZH2 与叉头盒 M1(FOXM1)结合,并与 FOXM1 靶基因的启动子结合。EZH2 PROTACs能诱导EZH2和FOXM1的降解,从而降低参与细胞周期进展和他莫昔芬抗性的靶基因的表达。总之,这项研究证明,EZH2 靶向 PROTACs 是未来治疗 BC(包括他莫昔芬耐药)的一条很有前景的研究途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
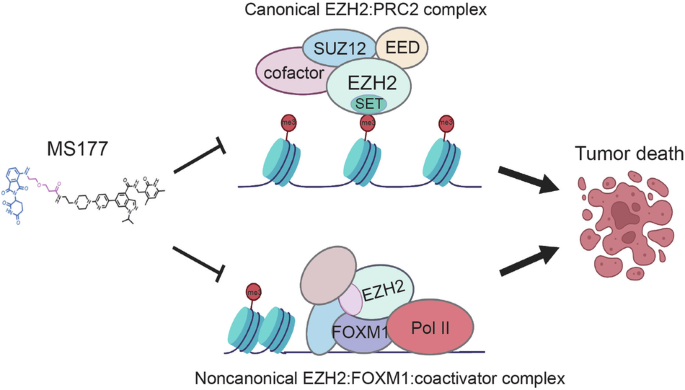
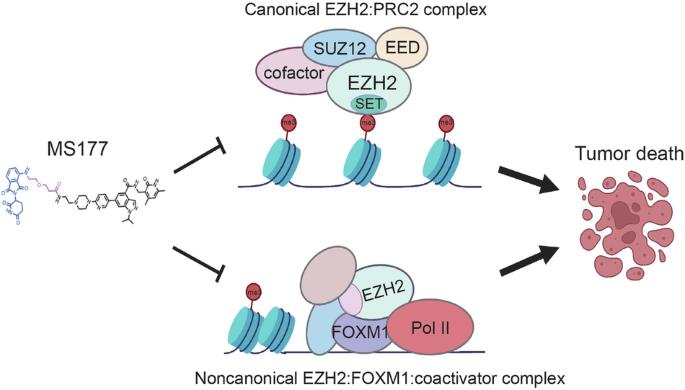
EZH2 PROTACs target EZH2- and FOXM1-associated oncogenic nodes, suppressing breast cancer cell growth
Breast cancer (BC) remains the second leading cause of cancer-related mortalities in women. Resistance to hormone therapies such as tamoxifen, an estrogen receptor (ER) inhibitor, is a major hurdle in the treatment of BC. Enhancer of zeste homolog 2 (EZH2), the methyltransferase component of the Polycomb repressive complex 2 (PRC2), has been implicated in tamoxifen resistance. Evidence suggests that EZH2 often functions noncanonically, in a methyltransferase-independent manner, as a transcription coactivator through interacting with oncogenic transcription factors. Unlike methyltransferase inhibitors, proteolysis targeting chimeras (PROTAC) can suppress both activating and repressive functions of EZH2. Here, we find that EZH2 PROTACs, MS177 and MS8815, effectively inhibited the growth of BC cells, including those with acquired tamoxifen resistance, to a much greater degree when compared to methyltransferase inhibitors. Mechanistically, EZH2 associates with forkhead box M1 (FOXM1) and binds to the promoters of FOXM1 target genes. EZH2 PROTACs induce degradation of both EZH2 and FOXM1, leading to reduced expression of target genes involved in cell cycle progression and tamoxifen resistance. Together, this study supports that EZH2-targeted PROTACs represent a promising avenue of research for the future treatment of BC, including in the setting of tamoxifen resistance.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


