RHNO1:DNA 复制压力、DNA 修复和癌症的十字路口。
IF 6.9
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
DNA 复制应激(DRS)反应是面对 DNA 复制的内在和外在障碍时维持基因组完整性的一种重要平衡机制。重要的是,肿瘤细胞中的 DRS 通常会显著增加,从而使肿瘤的生长和存活依赖于细胞的 DRS 反应。Rad9-Hus1-Rad1相互作用核孤儿1(RHNO1)是一种参与DRS反应的蛋白质,最近已成为癌症的潜在治疗靶点。RHNO1与9-1-1检查点钳夹和TopBP1相互作用,激活ATR/Chk1信号通路,这是DRS反应的关键介质。此外,最近还发现 RHNO1 是θ介导的末端连接(TMEJ)的关键促进因子,而 TMEJ 是一种 DNA 修复机制,与癌症进展和化疗耐药性有关。在这篇文献综述中,我们概述了目前对 RHNO1 的了解,包括其结构、在 DRS 反应中的功能以及在 DNA 修复中的作用,并讨论了其作为癌症治疗靶点的潜力。RHNO1 的治疗靶点有望用于 DRS 升高的肿瘤以及 DNA 修复缺陷的肿瘤,包括同源重组 DNA 修复缺陷(HRD)肿瘤。进一步研究 RHNO1 在癌症中的功能以及开发针对 RHNO1 的方法,有望产生新的癌症治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
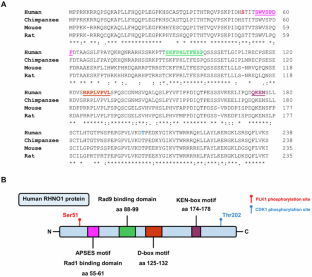
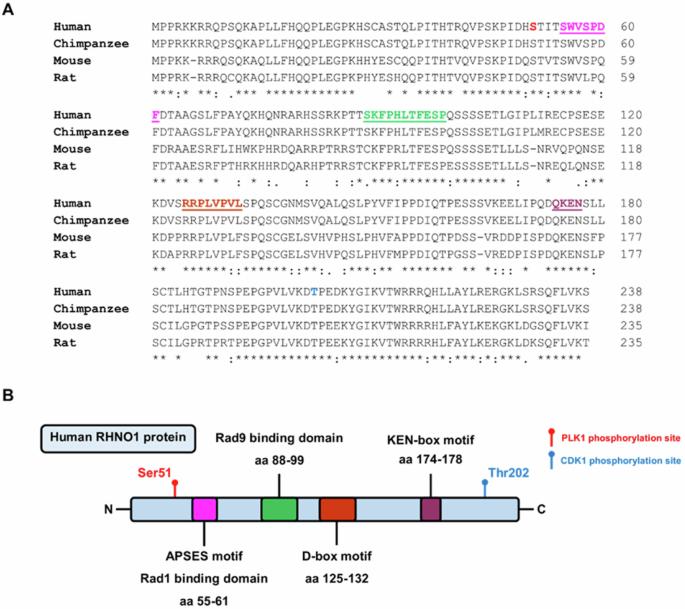
RHNO1: at the crossroads of DNA replication stress, DNA repair, and cancer
The DNA replication stress (DRS) response is a crucial homeostatic mechanism for maintaining genome integrity in the face of intrinsic and extrinsic barriers to DNA replication. Importantly, DRS is often significantly increased in tumor cells, making tumors dependent on the cellular DRS response for growth and survival. Rad9-Hus1-Rad1 Interacting Nuclear Orphan 1 (RHNO1), a protein involved in the DRS response, has recently emerged as a potential therapeutic target in cancer. RHNO1 interacts with the 9-1-1 checkpoint clamp and TopBP1 to activate the ATR/Chk1 signaling pathway, the crucial mediator of the DRS response. Moreover, RHNO1 was also recently identified as a key facilitator of theta-mediated end joining (TMEJ), a DNA repair mechanism implicated in cancer progression and chemoresistance. In this literature review, we provide an overview of our current understanding of RHNO1, including its structure, function in the DRS response, and role in DNA repair, and discuss its potential as a cancer therapeutic target. Therapeutic targeting of RHNO1 holds promise for tumors with elevated DRS as well as tumors with DNA repair deficiencies, including homologous recombination DNA repair deficient (HRD) tumors. Further investigation into RHNO1 function in cancer, and development of approaches to target RHNO1, are expected to yield novel strategies for cancer treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


