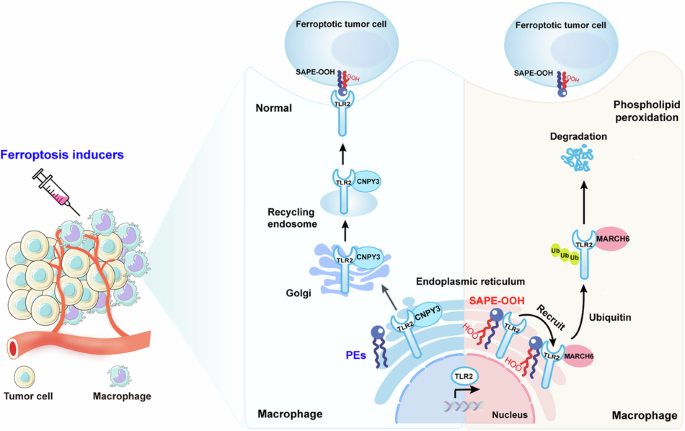
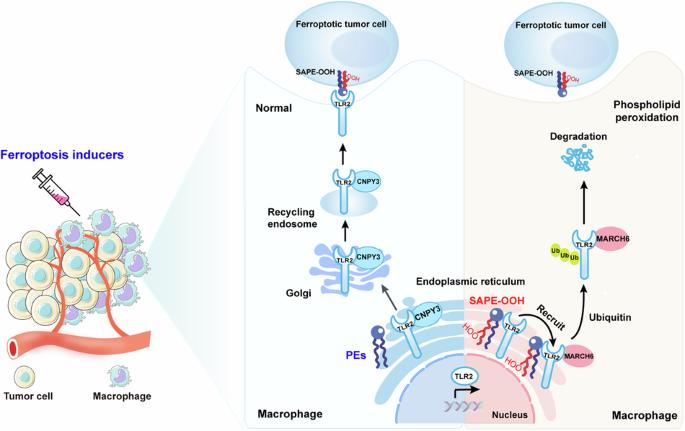
巨噬细胞中的磷脂过氧化反应可抑制对铁锈细胞的吞噬能力,从而产生抗肿瘤能力。
IF 13.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
铁氧化在癌症治疗中具有巨大的应用潜力。然而,铁突变诱导剂并非细胞特异性诱导剂,可导致肿瘤细胞和非肿瘤细胞的磷脂过氧化。这一局限性极大地限制了铁突变疗法作为一种安全有效的抗癌策略的应用。我们之前的研究表明,巨噬细胞可以通过 Toll 样受体 2(TLR2)吞噬铁氧体细胞。尽管取得了这一进展,但巨噬细胞中的磷脂过氧化影响其在使用铁氧体药物治疗肿瘤过程中的吞噬能力的确切机制仍不清楚。在这里,我们利用流式细胞分拣技术结合氧化还原磷脂组学确定了肿瘤微环境(TME)巨噬细胞中的磷脂过氧化反应会损害巨噬细胞通过吞噬作用消灭嗜铁肿瘤细胞的能力,最终导致肿瘤对嗜铁疗法产生耐药性。从机理上讲,巨噬细胞内质网(ER)中磷脂过氧化物的积累抑制了 TLR2 向质膜的迁移,并通过破坏 TLR2 与其伴侣 CNPY3 之间的相互作用使其滞留在 ER 中。随后,保留在ER中的TLR2招募E3连接酶MARCH6,并启动蛋白酶体依赖性降解。利用氧化还原磷脂组学,我们发现 1-steaoryl-2-15-HpETE-sn-glycero-3-phosphatidylethanolamine(SAPE-OOH)是这些效应的关键介质。最后,我们的发现阐明了巨噬细胞磷脂过氧化诱导的肿瘤抗铁治疗的新分子机制,并强调了TLR2-MARCH6轴是癌症治疗的潜在治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Phospholipid peroxidation in macrophage confers tumor resistance by suppressing phagocytic capability towards ferroptotic cells
Ferroptosis holds significant potential for application in cancer therapy. However, ferroptosis inducers are not cell-specific and can cause phospholipid peroxidation in both tumor and non-tumor cells. This limitation greatly restricts the use of ferroptosis therapy as a safe and effective anticancer strategy. Our previous study demonstrated that macrophages can engulf ferroptotic cells through Toll-like receptor 2 (TLR2). Despite this advancement, the precise mechanism by which phospholipid peroxidation in macrophages affects their phagocytotic capability during treatment of tumors with ferroptotic agents is still unknown. Here, we utilized flow sorting combined with redox phospholipidomics to determine that phospholipid peroxidation in tumor microenvironment (TME) macrophages impaired the macrophages ability to eliminate ferroptotic tumor cells by phagocytosis, ultimately fostering tumor resistance to ferroptosis therapy. Mechanistically, the accumulation of phospholipid peroxidation in the macrophage endoplasmic reticulum (ER) repressed TLR2 trafficking to the plasma membrane and caused its retention in the ER by disrupting the interaction between TLR2 and its chaperone CNPY3. Subsequently, this ER-retained TLR2 recruited E3 ligase MARCH6 and initiated the proteasome-dependent degradation. Using redox phospholipidomics, we identified 1-steaoryl-2-15-HpETE-sn-glycero-3-phosphatidylethanolamine (SAPE-OOH) as the crucial mediator of these effects. Conclusively, our discovery elucidates a novel molecular mechanism underlying macrophage phospholipid peroxidation-induced tumor resistance to ferroptosis therapy and highlights the TLR2-MARCH6 axis as a potential therapeutic target for cancer therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Death and Differentiation
生物-生化与分子生物学
CiteScore
24.70
自引率
1.60%
发文量
181
审稿时长
3 months
期刊介绍:
Mission, vision and values of Cell Death & Differentiation:
To devote itself to scientific excellence in the field of cell biology, molecular biology, and biochemistry of cell death and disease.
To provide a unified forum for scientists and clinical researchers
It is committed to the rapid publication of high quality original papers relating to these subjects, together with topical, usually solicited, reviews, meeting reports, editorial correspondence and occasional commentaries on controversial and scientifically informative issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


