研究促进己二酸二甲酯氢解的 Cu/ZnO 和 Cu/ZrO2 催化剂
IF 4.4
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
研究了两种载体(ZnO、ZrO2)和四种促进剂(Al2O3、ZnO、CoOx、NiO),以设计环保型铜基氢解催化剂。研究描述了催化剂的特性和在己二酸二甲酯氢解过程中的活性。虽然 ZrO2 改善了 CuO 纳米颗粒的还原性,但这些颗粒在反应条件下的稳定性较差。氧化锌则具有更好的稳定性,并减少了焦炭的形成。作为促进剂的 CoOx 增加了解离出的 H2 的表面可用性,并稳定了具有高表面积的 Cu 纳米粒子。相反,Al2O3 或 NiO 促进剂由于酸碱位点较多,既不能提高催化剂性能,也不能提高选择性。无论是用作支撑还是单一促进剂,氧化锌的重要作用都是由于铜-氧化锌协同作用增强了己二酸二甲酯的氢解活性,并提高了对己烷-1,6-二醇的理想选择性。总体而言,氧化锌支撑催化剂的氢解活性(TOFH)高出 5 倍。本文章由计算机程序翻译,如有差异,请以英文原文为准。
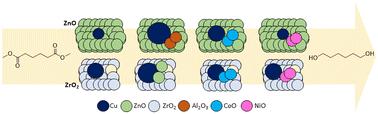
Study of promoted Cu/ZnO and Cu/ZrO2 catalysts for dimethyl adipate hydrogenolysis†
Two supports (ZnO, ZrO2) and four promoters (Al2O3, ZnO, CoOx, NiO) were investigated to design environmentally-friendly Cu-based hydrogenolysis catalysts. Both catalyst characterization and activity in dimethyl adipate hydrogenolysis were described. While ZrO2 improved the reducibility of CuO nanoparticles, these particles were less stable under reaction conditions. ZnO provided better stabilization and reduced coke formation. CoOx, used as a promoter, increased the surface availability of dissociated H2 and stabilized Cu nanoparticles with a high surface area. Conversely, Al2O3 or NiO promoters improved neither catalyst performance nor selectivity due to the higher number of acid–base sites. The essential role of ZnO, whether used as support or a single promoter, was attributed to Cu–ZnO synergy that enhanced the activity in dimethyl adipate hydrogenolysis and improved desired selectivity to hexane-1,6-diol. Overall, the hydrogenolysis activity (TOFH) was 5 times higher for ZnO-supported catalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


