基于荧光的 pH 值偏移测定法应用广泛,可用于酶挖掘和工程中酶活性的高通量测定
IF 4.4
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
许多对生物催化剂开发或作为药物靶点非常重要的酶在其催化反应过程中会因质子的释放或吸收而发生 pH 值的变化。在这里,我们展示了一种利用荧光 pH 指示剂 HPTS 开发的酶测定法,该方法可通过比率测量法(F485/F405)对在 5.5-8.5 可行 pH 范围内工作的多种 EC 类代表性酶进行可靠、连续的活性测定。使用该方法获得的动力学测量值与使用其他类型测定法确定的文献值非常吻合。此外,该测定法还被用于筛选来自嗜热地衣芽孢杆菌(Geobacillus stearothermophilus,TKgst)的转酮醇酶变体,旨在对 3-羟基醛进行底物杂合性和远端对映体选择性工程化。与基于吸收的转酮醇酶筛选检测方法相比,基于荧光的检测方法的灵敏度提高了 70 倍,检测限为 0.044 mM,Z 因子为 0.52。与 TKgst(L382F)碱基变体相比,G264 和 S385 位置的双位点突变产生的变体对测试的 3-羟基醛的活性提高了 5-15 倍。虽然在第一轮诱变中,定向进化工程策略并没有实现显著的远端对映体选择性,但基于荧光的简单 pH 值转移测定被证明是体外酶工程的多功能高通量初筛方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
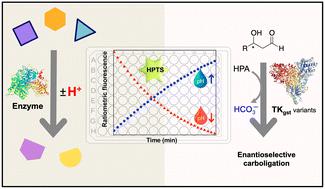
Fluorescence-based pH-shift assay with wide application scope for high-throughput determination of enzymatic activity in enzyme mining and engineering†‡
A number of enzymes important for biocatalyst development or as drug targets are associated with a pH shift during their catalytic reaction, owing to the concommitant release or uptake of protons. Here, we show that an enzyme assay developed using the fluorescent pH indicator HPTS can be adapted for reliable and continuous activity determination of representative enzymes from multiple EC classes that operate in the viable pH range 5.5–8.5, using ratiometric measurement (F485/F405). Kinetic measurements obtained with this method closely match literature values determined using other assay types. Further, the assay was employed to screen variants of transketolase from Geobacillus stearothermophilus (TKgst) aimed at engineering substrate promiscuity and remote enantioselectivity for 3-hydroxyaldehydes. The fluorescence-based assay displayed 70-fold improved sensitivity in comparison to an absorption-based assay for transketolase screening, with a limit of detection of 0.044 mM and Z-factor of 0.52. Double-site mutagenesis at the G264 and S385 positions yielded variants with 5–15-fold increased activity on the tested 3-hydroxyaldehydes compared to the TKgst (L382F) base variant. Although the directed evolution engineering strategy did not achieve significant remote enantioselectivity in this first round of mutagenesis, the simple fluorescence-based pH-shift assay was shown to be useful as a versatile primary high-throughput screen for in vitro enzyme engineering.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


