与锰(II)结合的欧芹 Rubisco 的结构。
IF 3.8
2区 化学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
光合作用的速率以及二氧化碳的固定速率受到核酮糖-1,5-二磷酸羧化酶/氧化酶(Rubisco)速率的限制。Rubisco 不仅催化率相对较低,而且在大亚基活性位点的金属特性方面也很杂乱。在自然界中,Rubisco 与 Mg(II)或 Mn(II)结合,这取决于叶绿体中这些金属离子的比例;对 Rubisco 的大多数研究都集中在与 Mg 结合的 Rubisco 上。在此,我们首次报告了与锰结合的 Rubisco 的晶体结构,并将其结构特性与其与镁结合的类似物进行了比较。本文章由计算机程序翻译,如有差异,请以英文原文为准。
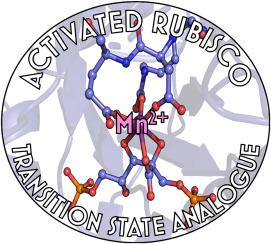
The structure of Mn(II)–bound Rubisco from Spinacia oleracea
The rate of photosynthesis and, thus, CO2 fixation, is limited by the rate of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco). Not only does Rubisco have a relatively low catalytic rate, but it also is promiscuous regarding the metal identity in the active site of the large subunit. In Nature, Rubisco binds either Mg(II) or Mn(II), depending on the chloroplastic ratio of these metal ions; most studies performed with Rubisco have focused on Mg-bound Rubisco. Herein, we report the first crystal structure of a Mn-bound Rubisco, and we compare its structural properties to those of its Mg-bound analogues.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
Journal of Inorganic Biochemistry
生物-生化与分子生物学
CiteScore
7.00
自引率
10.30%
发文量
336
审稿时长
41 days
期刊介绍:
The Journal of Inorganic Biochemistry is an established international forum for research in all aspects of Biological Inorganic Chemistry. Original papers of a high scientific level are published in the form of Articles (full length papers), Short Communications, Focused Reviews and Bioinorganic Methods. Topics include: the chemistry, structure and function of metalloenzymes; the interaction of inorganic ions and molecules with proteins and nucleic acids; the synthesis and properties of coordination complexes of biological interest including both structural and functional model systems; the function of metal- containing systems in the regulation of gene expression; the role of metals in medicine; the application of spectroscopic methods to determine the structure of metallobiomolecules; the preparation and characterization of metal-based biomaterials; and related systems. The emphasis of the Journal is on the structure and mechanism of action of metallobiomolecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


