八(2,6-氟苯基)卟吩在与含氮有机碱的酸碱反应中的反应活性
IF 0.8
4区 化学
Q4 CHEMISTRY, ORGANIC
引用次数: 0
摘要
摘要 研究了八(2,6-氟苯基)卟吩与吡啶、2-甲基吡啶、吗啉、哌啶、丁胺、叔丁胺、二乙胺和三乙胺在苯介质中的反应。大杂环与哌啶或丁胺之间的酸碱反应是一个缓慢的过程,会形成动力学上稳定的质子转移复合物。利用 CAM-B3LYP/cc-pVTZ 对这些复合物的结构进行了优化。分析了八(2,6-氟苯基)卟吩反应性的变化与含氮碱的立体结构和质子接受能力的函数关系。本文章由计算机程序翻译,如有差异,请以英文原文为准。
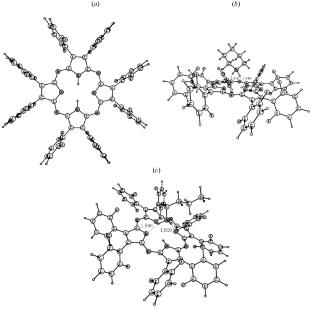

Reactivity of Octa(2,6-fluorophenyl)porphyrazine in Acid–Base Reactions with Nitrogenous Organic Bases
Reactions between octa(2,6-fluorophenyl)porphyrazine and pyridine, 2-methylpyridine, morpholine, piperidine, butylamine, tert-butylamine, diethylamine, and triethylamine in a benzene medium have been studied. The acid–base reactions between the macroheterocycle and piperidine or butylamine are slow processes leading to the formation of kinetically stable proton-transfer complexes. The structures of these complexes have been optimized using CAM-B3LYP/cc-pVTZ. The changes in the reactivity of octa(2,6-fluorophenyl)porphyrazine are analyzed as a function of the steric structure and proton-acceptor power of the nitrogenous base.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
1.40
自引率
25.00%
发文量
139
审稿时长
3-6 weeks
期刊介绍:
Russian Journal of Organic Chemistry is an international peer reviewed journal that covers all aspects of modern organic chemistry including organic synthesis, theoretical organic chemistry, structure and mechanism, and the application of organometallic compounds in organic synthesis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


