使用烷基硫代酸盐对 2-溴-α-去甲酰基酸衍生物进行化学和区域选择性去甲基化反应
IF 1.7
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们在此报告通过巯基亲核置换反应对 3,5-二甲氧基-2-溴-α-resorcylic 酸酯进行化学和区域选择性去甲基化的情况。经过优化的条件可使邻溴选择性脱甲基化 2-溴-α-resorcylic acid 的各种酯衍生物的收率高达 93%。我们的研究结果凸显了一种新型、高效、可靠的去甲基化试剂,以及一种有用的立体偏置引导策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
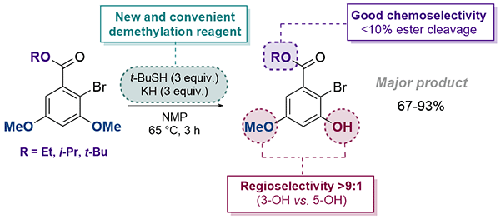
Chemo- and regioselective demethylation of 2-bromo-α-resorcylic acid derivatives using alkylthiolate salts
We report here the chemo- and regioselective demethylation of 3,5-dimethoxy-2-bromo-α-resorcylic acid esters by a thiolate nucleophilic displacement reaction. Optimized conditions facilitate yields up to 93% for o-bromo selective demethylation of diverse ester derivatives of dimethoxy 2-bromo-α-resorcylic acid. Our results highlight a new, efficient, and reliable demethylation reagent, as well as a useful steric bias directing strategy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Synlett
化学-有机化学
CiteScore
3.40
自引率
5.00%
发文量
369
审稿时长
1 months
期刊介绍:
SYNLETT is an international journal reporting research results and current trends in chemical synthesis in short personalized reviews and preliminary communications. It covers all fields of scientific endeavor that involve organic synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines and offers the possibility to publish scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


