关于合成可乐丽酰胺 A 的研究:构建分子的 C19-C30 段
IF 1.7
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
通过在片段 9 和 10 之间进行 Sonogashira 偶联,合成了具有神经保护作用的天然产物可乐三色酰胺 A 的 C19-C30 片段。片段 9 的关键步骤是 Maruoka-Keck烯丙基化反应、Evans syn-aldol 反应和 Takai 烯化反应,而片段 10 的关键步骤是糖苷化反应和 Ohira-Bestmann 反应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
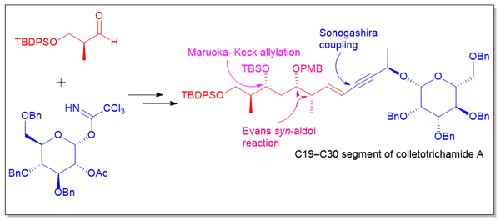
Studies toward the synthesis of Colletotrichamide A: Construction of C19-C30 Segment of the Molecule
The synthesis of C19–C30 segment of the neuroprotective natural product colletotrichamide A has been achieved by performing Sonogashira coupling between the fragments 9 and 10. Maruoka-Keck allylation, Evans syn-aldol and Takai olefinations were used as the key steps for fragment 9 whereas glycosidation and Ohira-Bestmann reactions were used as the pivotal steps for accessing fragment 10.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Synlett
化学-有机化学
CiteScore
3.40
自引率
5.00%
发文量
369
审稿时长
1 months
期刊介绍:
SYNLETT is an international journal reporting research results and current trends in chemical synthesis in short personalized reviews and preliminary communications. It covers all fields of scientific endeavor that involve organic synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines and offers the possibility to publish scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


