NNMT 通过表观遗传调控 ETS2/VEGFA 轴,改变癌症相关成纤维细胞的促血管生成表型。
IF 6.9
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
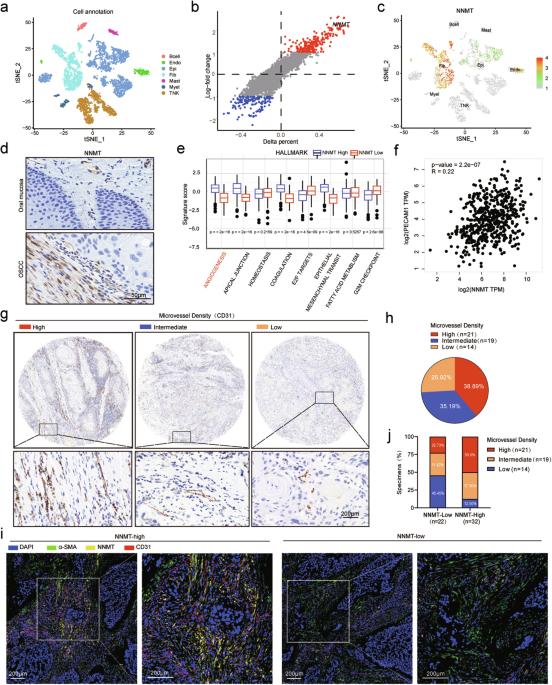
众所周知,癌症相关成纤维细胞(CAFs)可促进口腔鳞状细胞癌(OSCC)的血管生成。然而,CAFs在肿瘤微环境中促进血管生成的表观遗传学机制仍鲜为人知。研究发现,N-甲基转移酶家族成员烟酰胺 N'-甲基转移酶(NNMT)是激活 CAFs 的关键分子。这项研究表明,成纤维细胞中的NNMT通过表观遗传重编程-ETS2-VEGFA信号轴促进了OSCC中的血管生成和肿瘤生长。单细胞RNA测序(scRNA-seq)分析表明,NNMT主要在头颈部鳞状细胞癌(HNSCC)成纤维细胞中高表达。此外,对 TCGA 数据库和临床样本的多重免疫组化染色分析也发现 NNMT 与肿瘤血管生成之间存在正相关。该研究进一步采用了组装类器官模型和成纤维细胞-内皮细胞共培养模型来验证NNMT的促血管生成能力。在分子水平上,研究发现NNMT在CAFs中的高表达可通过介导甲基化沉积调节H3K27甲基化水平,从而促进ETS2的表达。此外,本研究还证实 ETS2 是 VEGFA 的激活转录因子。总之,我们的研究结果勾勒出了血管生成的表观遗传分子调控网络,为探索OSCC的新靶点和临床策略提供了理论依据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
NNMT switches the proangiogenic phenotype of cancer-associated fibroblasts via epigenetically regulating ETS2/VEGFA axis
Cancer-associated fibroblasts (CAFs) are known to promote angiogenesis in oral squamous cell carcinoma (OSCC). However, the epigenetic mechanisms through which CAFs facilitate angiogenesis within the tumor microenvironment are still poorly characterized. Nicotinamide N’-methyltransferase (NNMT), a member of the N-methyltransferase family, was found to be a key molecule in the activation of CAFs. This study shows that NNMT in fibroblasts contributes to angiogenesis and tumor growth through an epigenetic reprogramming-ETS2-VEGFA signaling axis in OSCC. Single-cell RNA Sequencing (scRNA-seq) analysis suggests that NNMT is mainly highly expressed in fibroblasts of head and neck squamous cell carcinoma (HNSCC). Moreover, analysis of the TCGA database and multiple immunohistochemical staining of clinical samples also identified a positive correlation between NNMT and tumor angiogenesis. This research further employed an assembled organoid model and a fibroblast-endothelial cell co-culture model to authenticate the proangiogenic ability of NNMT. At the molecular level, high expression of NNMT in CAFs was found to promote ETS2 expression by regulating H3K27 methylation level through mediating methylation deposition. Furthermore, ETS2 was verified to be an activating transcription factor of VEGFA in this study. Collectively, our findings delineate an epigenetic molecular regulatory network of angiogenesis and provide a theoretical basis for exploring new targets and clinical strategy in OSCC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


